Pyruvate kinase M2 (PKM2), the rate-limiting enzyme of glycolysis, plays a critical role in macrophage activation and a broad spectrum of chronic liver diseases. However, whether PKM2 contributes to the pathogenesis of acute liver injury (ALI) remains largely unexplored.
PKM2 expression was assessed in human and mouse ALI livers. Macrophage-specific PKM2 knockout mice were challenged by two independent ALI models, induced by acetaminophen (APAP) and lipopolysaccharide/D-galactosamine (LPS/D-GalN), to explore the role and regulatory mechanism of macrophage PKM2 in ALI progression.
By bioinformatic screening and analysis of ALI liver, we found that PKM2 was significantly upregulated in the liver tissues of ALI patients and mice. Immunofluorescence staining further demonstrated that PKM2 was markedly upregulated in macrophages during ALI progression. Notably, macrophage PKM2 depletion effectively alleviated APAP- and LPS/D-GalN-induced ALI, as demonstrated by ameliorated immune cells infiltration, pro-inflammatory mediators, and hepatocellular cell death. PKM2-deficient macrophages showed M2 anti-inflammatory polarization in vivo and in vitro. Furthermore, PKM2 deletion limited HIF-1α signaling and aerobic glycolysis of macrophages, which thereby attenuated macrophage pro-inflammatory activation and hepatocyte injury. Pharmacological PKM2 antagonist efficiently ameliorated liver injury and prolonged the survival of mice in APAP-induced ALI model.
Our study highlights the pivotal role of macrophage PKM2 in advancing ALI, and therapeutic targeting of PKM2 may serve as a novel strategy to combat ALI.
Copyright © 2025 Kang, Xie, Cui, Chen, Xie, Li, Lv, Ye, Zhao, Zhang, Hong and Qu.
Product Citations: 194
Macrophage PKM2 depletion ameliorates hepatic inflammation and acute liver injury in mice.
In Frontiers in Pharmacology on 12 May 2025 by Kang, Z., Xie, R., et al.
-
FC/FACS
-
Mus musculus (House mouse)
-
Immunology and Microbiology
-
Pharmacology
In International Journal of Nanomedicine on 23 April 2025 by Chen, W., Zhang, Y., et al.
Pulmonary microvascular endothelial cells (PMVECs) are notably implicated in the pathogenesis of sepsis-induced lung injury. Exosomes derived from PMVECs facilitate intercellular communication among various cell types, especially crosstalk with macrophages. Heme oxygenase-1 (HO-1), an early stress-responsive enzyme with inherent protective functions, has been implicated in acute lung injury (ALI) mitigation. But research on the mechanism of HO-1 in macrophage polarization via PMVEC exosomes in sepsis-induced lung injury is lacking.
To investigate the role of HO-1 in the interaction between endothelial cells and macrophages, HO-1 knockout mouse model were established. Exosomes from PMVECs were isolated, and differential expression of microRNA (miRNA) was determined by sequencing. An in vitro co-culture system involving Murine Alveolar Macrophage Cell Line (MH-S cells) and HO-1/ PMVECs-derived exosomes (HP-exos) was used to investigate the underlying mechanisms. To further verify the involvement of HO-1 in intercellular communication through exosomal miRNA in vivo, the level of pulmonary inflammation was evaluated, and the polarization of pulmonary macrophages was analyzed.
The results showed that miR-184-3p was significantly downregulated in HP-exos, and supplementation of miR-184-3p enhanced the polarization of M1 macrophages, thus intensifying lung inflammation. HO-1 regulates the polarization of macrophages by regulating endothelial exosomes. Overexpression of HO-1 downregulates miR-184-3p, which negatively regulates Semaphorin 7A (Sema7a), which attenuated M1 type macrophages (M1) polarization and augmented M2 type macrophages (M2) polarization, thereby partially mitigating lung injury and inflammation.
Collectively, we elucidated a novel potential therapeutic mechanism that HO-1 alleviate inflammation by modulating the M1/M2 ratio in sepsis-induced ALI by regulating miR-184-3p/Sema7a expression.
© 2025 Chen et al.
-
Immunology and Microbiology
In Frontiers in Neurology on 17 April 2025 by Bae, S. H., Ikezono, T., et al.
Cochlin is the most abundant protein in the inner ear. The cleaved N-terminal domain of cochlin, known as LCCL and referred to as CTP (cochlin tomoprotein) in clinical biomarker usage, is a perilymph-specific protein and is widely used as a biomarker to detect perilymph leakage. However, little is known about the secretion or presence of LCCL in the middle ear, even though it can result in false positives when using LCCL as a biomarker for perilymph leakage.
We conducted translational research in humans and mice. A retrospective observational study was conducted on human patients who underwent multiple CTP tests after tympanostomy. In parallel, an experimental study on cochlin and its cleaved product, LCCL, was performed in mice.
We found the exceptionally elevated level of CTP within 10 days after tympanostomy in humans regardless of the presence of definite perilymph leakage. In addition, we identified LCCL in the middle ear after tympanostomy in mice. The concentration of LCCL peaked at three days post-tympanic injury. Importantly, the origin of LCCL in the middle ear lavage was not from the inner ear but is secreted from the middle ear space especially the annular ligament, suggesting it functions as an innate immune response in the middle ear.
Tympanostomy for the CTP test results in a false positive when the sampling is delayed. While LCCL is a reliable biomarker for clinically detecting perilymph fistula, the timing of its application should be carefully considered to avoid false-positive results.
Copyright © 2025 Bae, Ikezono, Park, Kim, Matsumura, Saito, Maeda, Matsuda and Jung.
-
IHC-IF
-
Mus musculus (House mouse)
Mutant CEBPA promotes tolerance to inflammatory stress through deficient AP-1 activation.
In Nature Communications on 12 April 2025 by Cadefau-Fabregat, M., Martínez-Cebrián, G., et al.
The CEBPA transcription factor is frequently mutated in acute myeloid leukemia (AML). Mutations in the CEBPA gene, which are typically biallelic, result in the production of a shorter isoform known as p30. Both the canonical 42-kDa isoform (p42) and the AML-associated p30 isoform bind chromatin and activate transcription, but the specific transcriptional programs controlled by each protein and how they are linked to a selective advantage in AML is not well understood. Here, we show that cells expressing the AML-associated p30 have reduced baseline inflammatory gene expression and display altered dynamics of transcriptional induction in response to LPS, consequently impacting cytokine secretion. This confers p30-expressing cells an increased resistance to the adverse effects of prolonged exposure to inflammatory signals. Mechanistically, we show that these differences primarily arise from the differential regulation of AP-1 family proteins. In addition, we find that the impaired function of the AP-1 member ATF4 in p30-expressing cells alters their response to ER stress. Collectively, these findings uncover a link between mutant CEBPA, inflammation and the stress response, potentially revealing a vulnerability in AML.
© 2025. The Author(s).
-
Immunology and Microbiology
In ACS Nano on 8 April 2025 by Mo, X., shen, A., et al.
Macrophage plays critical roles in immune-related diseases, acting as a crucial therapeutic target for immunotherapy. Rational design and development of effective therapeutics for macrophage reprogramming are still challenging. Here, we rationally engineered polysaccharide nanoadjuvants to reprogram macrophage functions for enhanced immunotherapy in multiple diseases through a macrophage phenotype-specific nanoprobe (MPSNPr)-assisted high-throughput phenotypic screen. This MPSNPr exhibited high macrophage M1 phenotype specificity because of the formation of H-aggregates on the outer surface and the binding to glucose transporter 1 receptors by the polysaccharide nanocarrier. Based on this MPSNPr, a high-throughput platform was constructed and employed to screen a variety of pharmaceuticals for macrophage reprogramming, being able to identify both pro-inflammatory and anti-inflammatory drug candidates. Polysaccharide nanoadjuvants, Dex-BA and Dex-SAL, were rationally engineered with two potent candidates to amplify macrophage reprogramming efficacy both in vitro and in vivo. Dex-BA significantly inhibited tumor growth by inducing macrophage M1 polarization, dendritic cell maturation, and cytotoxic T cell activation in a mice melanoma model. Dex-SAL alleviated rheumatoid arthritis symptoms with reduced inflammation by reprogramming activated macrophages toward anti-inflammatory phenotype. Our work provides a robust strategy for the rational design and development of effective therapeutics for enhanced macrophage-mediated immunotherapy in diverse diseases.
-
Cancer Research
-
Immunology and Microbiology
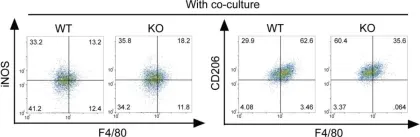
In Theranostics on 7 January 2020 by Zhao, S. J., Kong, F. Q., et al.
Fig.4.C

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Theranostics by CiteAb, provided under a CC-BY license
Image 1 of 3
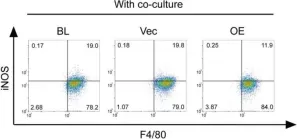
In Theranostics on 7 January 2020 by Zhao, S. J., Kong, F. Q., et al.
Fig.5.E

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Theranostics by CiteAb, provided under a CC-BY license
Image 1 of 3
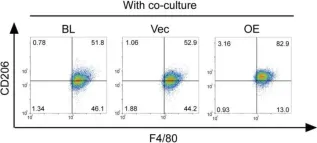
In Theranostics on 7 January 2020 by Zhao, S. J., Kong, F. Q., et al.
Fig.5.G

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from Theranostics by CiteAb, provided under a CC-BY license
Image 1 of 3