The CD47/SIRPα axis conveys a 'don't eat me' signal, thereby thwarting the phagocytic clearance of tumor cells. Although blocking antibodies targeting CD47 have demonstrated promising anti-tumor effects in preclinical models, clinical trials involving human cancer patients have not yielded ideal results. Exploring the regulatory mechanisms of CD47 is imperative for devising more efficacious combinational therapies. Here, we report that inhibiting USP2 prompts CD47 degradation and reshapes the tumor microenvironment (TME), thereby enhancing anti-PD-1 immunotherapy. Mechanistically, USP2 interacts with CD47, stabilizing it through deubiquitination. USP2 inhibition destabilizes CD47, thereby boosting macrophage phagocytosis. Single-cell RNA sequencing shows USP2 inhibition reprograms TME, evidenced by increasing M1 macrophages and CD8+ T cells while reducing M2 macrophages. Combining ML364 with anti-PD-1 reduces tumor burden in mouse models. Clinically, low USP2 expression predicts a better response to anti-PD-1 treatment. Our findings uncover the regulatory mechanism of CD47 by USP2 and targeting this axis boosts anti-tumor immunity.
© 2025. The Author(s).
Product Citations: 121
USP2 inhibition unleashes CD47-restrained phagocytosis and enhances anti-tumor immunity.
In Nature Communications on 16 May 2025 by Dai, P., Sun, Y., et al.
-
Cancer Research
-
Immunology and Microbiology
In JCI Insight on 8 May 2025 by Xu, S., Li, H., et al.
The inflammatory response after myocardial infarction (MI) is a precisely regulated process that greatly affects subsequent wound healing and remodeling. However, understanding about the process is still limited. Macrophages are critically involved in inflammation resolution after MI. Krüppel-like factor 9 (Klf9) is a C2H2 zinc finger-containing transcription factor that has been implicated in glucocorticoid regulation of macrophages. However, the contribution of Klf9 to macrophage phenotype and function in the context of MI remains unclear. Our study revealed that KLF9 deficiency resulted in higher mortality and cardiac rupture rate, as well as a considerable exacerbation in cardiac function. Single-cell RNA sequencing and flow cytometry analyses revealed that, compared with WT mice, Klf9-/- mice displayed excessive neutrophil infiltration, insufficient macrophage infiltration, and a reduced proportion of monocyte-derived CD206+ macrophages after MI. Moreover, the expression of IFN-γ/STAT1 pathway genes in Klf9-/- cardiac macrophages was dysregulated, characterized by insufficient expression at 1 day post-MI and excessive expression at day 3 post-MI. Mechanistically, Klf9 directly binds to the promoters of Stat1 gene, regulating its transcription. Overall, these findings indicate that Klf9 beneficially influences wound healing after MI by modulating macrophage recruitment and differentiation by regulating the IFN-γ/STAT1 signaling pathway.
-
FC/FACS
-
Mus musculus (House mouse)
-
Cardiovascular biology
-
Immunology and Microbiology
In Nature Communications on 21 April 2025 by Yan, J., Wang, S. Y., et al.
Idiopathic pulmonary fibrosis (IPF) is a severe lung disease occurring throughout the world; however, few clinical therapies are available for treating this disorder. Overactivated fibroblasts drive abnormal fibrosis accumulation to maintain dynamic balance between inflammation and extracellular matrix (ECM) stiffness. Given pulmonary cell can regenerate, the lung may possess self-repairing abilities if fibrosis is removed via clearance of overactivated fibroblasts. The aim of this study was to evaluate the therapeutic activity of transient antifibrotic chimeric antigen receptor (CAR) T cells (generated via a novelly-designed lipid nanoparticle-messenger RNA (LNP-mRNA) system) and explore the regeneration mechanisms of lung in a male mouse model of bleomycin-induced pulmonary fibrosis. Here we found that fibrosis-induced ECM stiffening impaired alveolar epithelial cell compensation. The proposed LNP-mRNA therapy eliminated overactivated fibroblasts to rescue pulmonary fibrosis. The restored ECM environment regulated the cellular profile. The elevated plasticity of AT2 and Pclaf+ cells increased AT1 cell population via polarization. Apoe+ macrophages and increased numbers of effector T cells were shown to reestablish pulmonary immunity. Hence, LNP-mRNA treatment for fibrosis can restore pulmonary structure and function to similar degrees to those of a healthy lung. This therapy is a potential treatment for IPF patients.
© 2025. The Author(s).
-
Cardiovascular biology
-
Immunology and Microbiology
In Frontiers in Immunology on 7 April 2025 by Chen, L., Ruan, G., et al.
Immune checkpoint therapy for colorectal cancer (CRC) has been found to be unsatisfactory for clinical treatment. Fecal microbiota transplantation (FMT) has been shown to remodel the intestinal flora, which may improve the therapeutic effect of αPD-1. Further exploration of key genera that can sensitize cells to αPD-1 for CRC treatment and preliminary exploration of immunological mechanisms may provide effective guidance for the clinical treatment of CRC.
In this study, 16S rRNA gene sequencing was analyzed in the fecal flora of both responders and no-responders to αPD-1 treatment, and the therapeutic effect was experimentally verified.
Pseudomonas aeruginosa was found to be highly abundant in the fecal flora of treated mice, and Pseudomonas aeruginosa mannose-sensitive hemagglutinin (PA-MSHA) in combination with αPD-1 was effective in the treatment of CRC through the induction of CD8+ T-cell immunological effects.
The clinical drug PA-MSHA can be used in combination with αPD-1 for the treatment of CRC as a potential clinical therapeutic option.
Copyright © 2025 Chen, Ruan, Zhao, Yi, Xiao, Tian, Cheng, Chen and Wei.
-
Cancer Research
-
Immunology and Microbiology
Preprint on Research Square on 20 March 2025 by Wang, Y., Moura, A. K., et al.
Abstract Niemann-Pick Disease (NPD) is a rare autosomal recessive lysosomal storage disorder (LSD) caused by the deficiency of acid sphingomyelinase (ASMD), which is encoded by the Smpd1 gene. ASMD impacts multiple organ systems in the body, including the cardiovascular system. This study is the first to characterize cardiac pathological changes in ASMD mice under baseline conditions, offering novel insights into the cardiac implications of NPD. Using histological analysis, biochemical assays, and echocardiography, we assessed cardiac pathological changes and function in Smpd1−/− mice compared to Smpd1+/+ littermate controls. Immunofluorescence and biochemical assays demonstrated that ASMD induced lysosomal dysfunction, as evidenced by the accumulation of lysosomal-associated membrane proteins, lysosomal protease, and autophagosomes in pericytes and cardiomyocytes. This lysosomal dysfunction was accompanied by pericytes and cardiomyocytes inflammation, characterized by increased expression of caspase1 and inflammatory cytokines, and infiltration of inflammatory cells in the cardiac tissues of Smpd1−/− mice. In addition, histological analysis revealed increased lipid deposition and cardiac steatosis, along with pericyte-to-myofibroblast transition (PMT) and interstitial fibrosis in Smpd1−/− mice. Moreover, echocardiography further demonstrated that Smpd1−/− mice developed coronary microvascular dysfunction (CMD), as evidenced by decreased coronary blood flow velocity and increased coronary arteriolar wall thickness. Additionally, these mice exhibited significant impairments in systolic and diastolic cardiac function, as shown by a reduced ejection fraction and prolonged left ventricular relaxation time constant (Tau value). These findings suggest that ASMD induces profound pathological changes and vascular dysfunction in the myocardium, potentially driven by mechanisms involving lysosomal dysfunction as well as both pericytes and cardiac inflammation.
-
Cardiovascular biology
-
Cell Biology
In J Transl Med on 10 April 2023 by Lin, Z., Lv, Z., et al.
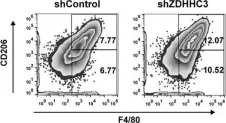
Fig.6.H

-
FC/FACS
-
Mus musculus (House mouse)
Collected and cropped from J Transl Med by CiteAb, provided under a CC-BY license
Image 1 of 1