Cancer driver mutations often show distinct temporal acquisition patterns, but the biological basis for this, if any, remains unknown. RAS mutations occur invariably late in the course of acute myeloid leukaemia, upon progression or relapsed/refractory disease1-6. Here, by using human leukaemogenesis models, we first show that RAS mutations are obligatory late events that need to succeed earlier cooperating mutations. We provide the mechanistic explanation for this in a requirement for mutant RAS to specifically transform committed progenitors of the myelomonocytic lineage (granulocyte-monocyte progenitors) harbouring previously acquired driver mutations, showing that advanced leukaemic clones can originate from a different cell type in the haematopoietic hierarchy than ancestral clones. Furthermore, we demonstrate that RAS-mutant leukaemia stem cells (LSCs) give rise to monocytic disease, as observed frequently in patients with poor responses to treatment with the BCL2 inhibitor venetoclax. We show that this is because RAS-mutant LSCs, in contrast to RAS-wild-type LSCs, have altered BCL2 family gene expression and are resistant to venetoclax, driving clinical resistance and relapse with monocytic features. Our findings demonstrate that a specific genetic driver shapes the non-genetic cellular hierarchy of acute myeloid leukaemia by imposing a specific LSC target cell restriction and critically affects therapeutic outcomes in patients.
© 2024. The Author(s).
Product Citations: 14
RAS-mutant leukaemia stem cells drive clinical resistance to venetoclax.
In Nature on 1 December 2024 by Sango, J., Carcamo, S., et al.
-
FC/FACS
-
Stem Cells and Developmental Biology
In Clinical and Translational Medicine on 1 August 2024 by Wang, D., Li, S., et al.
Chronic obstructive pulmonary disease (COPD) contributes to the incidence and prognosis of lung cancer. The presence of COPD significantly increases the risk of lung squamous cell carcinoma (LSCC). COPD may promote an immunosuppressive microenvironment in LSCC by regulating the expression of immune-inhibitory factors in T cells, although the mechanisms remain unclear. In this study, we aimed to decipher the tumour microenvironment signature for LSCC with COPD at a single-cell level.
We performed single-cell RNA sequencing on tumour tissues from LSCC with or without COPD, then investigated the features of the immune and tumour cells. We employed multiple techniques, including multispectral imaging, flow cytometry, tissue microarray analysis, survival analysis, co-culture systems and in vitro and in vivo treatment experiments, to validate the findings obtained from single-cell analyses.
LSCC with COPD showed increased proportions of tumour-associated macrophages (TAMs) and higher levels of CD8+ T cell exhaustion molecules, which contributed to an immunosuppressive microenvironment. Further analysis revealed a critical cluster of CD74+ tumour cells that expressed both epithelial and immune cell signatures, exhibited a stronger capacity for tumorigenesis and predicted worse overall survival. Notably, migration inhibitory factor (MIF) secreted by TAMs from LSCC with COPD may promote the activation of CD74. MIF-CD74 may interact with CD8+ T cells and impair their anti-tumour activity by regulating the PI3K-STAT3-programmed cell death-1 ligand 1 signalling pathway, facilitating tumour proliferation and immune evasion.
Our comprehensive picture of the tumour ecosystem in LSCC with COPD provides deeper insights into relevant immune evasion mechanisms and potential targets for immunotherapy.
Our results demonstrated higher proportions of tumour-associated macrophages (TAMs) and higher levels of exhaustion molecules in CD8+ T cells in the microenvironment of LSCC with COPD. CD74+tumour cells were associated with poor disease prognosis. Migration inhibitory factor (MIF)-CD74 may interact with CD8+ T cells and impair their anti-tumour activity by regulating the PI3K-STAT3-PD-L1 signalling pathway, facilitating immune evasion.
© 2024 The Author(s). Clinical and Translational Medicine published by John Wiley & Sons Australia, Ltd on behalf of Shanghai Institute of Clinical Bioinformatics.
-
Cancer Research
-
Cardiovascular biology
-
Immunology and Microbiology
In Cancers on 27 October 2023 by Kwiecień, I., Rutkowska, E., et al.
Macrophages play an important role in the suppression and activation of immune anti-cancer response, but little is known about dominant macrophage phenotype in the lung cancer environment, evaluated by bronchoalveolar lavage fluid (BALF). The aim of this study was to characterize macrophages in BALF from a lung affected by cancer (cBALF) and a healthy lung (hBALF) of the same patient regarding their individual macrophage polarization and selected cytokines profile. A total of 36 patients with confirmed lung cancer were investigated. Macrophages markers: CD206 CD163 CD80 CD86 CD40 CD45, Arginase-1, and CD68 were evaluated by flow cytometry. Cytokines (IL-1 RA, IL-6, IL-10, TNF-α, IL-1β, IL-12, IL-23, and TGF-β) profile was analyzed. There was higher median proportion of macrophages in Cbalf than in Hbalf. The population of macrophages presented immunophenotype: Ccd68+bright CD206+bright CD163+bright CD80+ CD86+ CD40+bright CD45+ cArginase+. We observed some trends in the expression of the analyzed antigens in clBALF and hlBLAF. The highest concentrations of IL-1RA and IL-6 were in Cbalf and Hbalf supernatant. There were the correlations between pro- and anti-inflammatory cytokines. The findings showed that macrophages include a diverse and plastic group with the presence of different antigens and cytokines, and determining the target phenotype is a complex and variable process.
-
FC/FACS
-
Homo sapiens (Human)
-
Cancer Research
In Cell Reports Medicine on 17 October 2023 by Tang, R., Xu, J., et al.
The molecular dynamics of pancreatic ductal adenocarcinoma (PDAC) under chemotherapy remain incompletely understood. The widespread use of neoadjuvant chemotherapy (NAC) provides a unique opportunity to investigate PDAC samples post-chemotherapy. Leveraging a cohort from Fudan University Shanghai Cancer Center, encompassing PDAC samples with and without exposure to neoadjuvant albumin-bound paclitaxel and gemcitabine (AG), we have compiled data from single-cell and spatial transcriptomes, proteomes, bulk transcriptomes, and metabolomes, deepening our comprehension of the molecular changes in PDACs in response to chemotherapy. Metabolic flux analysis reveals that NAC induces a reprogramming of PDAC metabolic patterns and enhances immunogenicity. Notably, NAC leads to the downregulation of glycolysis and the upregulation of CD36. Tissue microarray analysis demonstrates that high CD36 expression is linked to poorer survival in patients receiving postoperative AG. Targeting CD36 synergistically improves the PDAC response to AG both in vitro and in vivo, including patient-derived preclinical models.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
-
Biochemistry and Molecular biology
-
Cancer Research
-
Cell Biology
-
Immunology and Microbiology
Tumor-associated macrophages trigger MAIT cell dysfunction at the HCC invasive margin.
In Cell on 17 August 2023 by Ruf, B., Bruhns, M., et al.
Mucosal-associated invariant T (MAIT) cells represent an abundant innate-like T cell subtype in the human liver. MAIT cells are assigned crucial roles in regulating immunity and inflammation, yet their role in liver cancer remains elusive. Here, we present a MAIT cell-centered profiling of hepatocellular carcinoma (HCC) using scRNA-seq, flow cytometry, and co-detection by indexing (CODEX) imaging of paired patient samples. These analyses highlight the heterogeneity and dysfunctionality of MAIT cells in HCC and their defective capacity to infiltrate liver tumors. Machine-learning tools were used to dissect the spatial cellular interaction network within the MAIT cell neighborhood. Co-localization in the adjacent liver and interaction between niche-occupying CSF1R+PD-L1+ tumor-associated macrophages (TAMs) and MAIT cells was identified as a key regulatory element of MAIT cell dysfunction. Perturbation of this cell-cell interaction in ex vivo co-culture studies using patient samples and murine models reinvigorated MAIT cell cytotoxicity. These studies suggest that aPD-1/aPD-L1 therapies target MAIT cells in HCC patients.
Published by Elsevier Inc.
-
Cancer Research
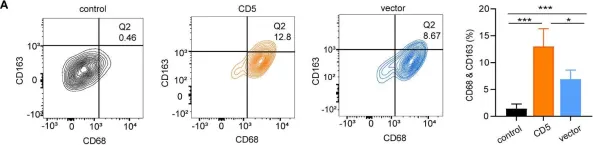
In Front Oncol on 25 October 2022 by Liu, M. K., Cheng, L. L., et al.
Fig.5.A

-
FC/FACS
-
Collected and cropped from Front Oncol by CiteAb, provided under a CC-BY license
Image 1 of 1