Inflammatory bowel disease (IBD) is a relapsing disorder characterized by uncontrolled chronic inflammation of the gastrointestinal tract, posing a significant therapeutic challenge owing to the limited efficacy and undesirable side effects of current therapeutic options. A key pathological hallmark of IBD is the excessive production of reactive oxygen species (ROS). Hence, therapeutic strategies aimed at reducing ROS levels are promising for relieving these inflammatory conditions. Vitamin C-a natural nutrient for the human body-is well known for its potent antioxidant effects. However, the clinical development of vitamin C as a therapeutic drug has been hindered by its poor stability, rapid metabolism, and inadequate tissue accumulation. Herein, we report that the bioavailability of vitamin C can be enhanced by chemically reprogramming it with a small panel of long-chain fatty acids that aid in the aqueous self-assembly of the resulting drug conjugates to create self-deliverable nanoassemblies, enhancing their inflammation disease-oriented delivery and cellular uptake. In mice with dextran sulfate sodium-induced colitis, the optimal vitamin C-lipid nanoassemblies preferentially accumulated in inflamed colonic tissues following systemic administration and substantially ameliorated disease severity. We extended this strategy to incorporate the clinically approved glucocorticoid budesonide into the vitamin C nanosystem, facilitating a synergistic combination. In the chronic colitis model, the combination treatment reduced inflammation without compromising global immunity. Mechanistically, the treatment modulated the intestinal inflammatory microenvironment and altered the immune cell landscape, partly through regulation of the gut microbiome. Given its anticipated negligible side effects, this novel nanoassembly platform leveraging small-molecule lipidation may become a promising therapeutic for treating various inflammatory diseases.
© 2025. The Author(s).
Product Citations: 28
In Journal of Nanobiotechnology on 25 March 2025 by Xian, S., Meng, F., et al.
-
FC/FACS
-
Mus musculus (House mouse)
Stroma-derived Dickkopf-1 contributes to the suppression of NK cell cytotoxicity in breast cancer.
In Nature Communications on 30 January 2025 by Lee, S., Ricci, B., et al.
Mechanisms related to tumor evasion from NK cell-mediated immune surveillance remain enigmatic. Dickkopf-1 (DKK1) is a Wnt/β-catenin inhibitor, whose levels correlate with breast cancer progression. We find DKK1 to be expressed by tumor cells and cancer-associated fibroblasts (CAFs) in patient samples and orthotopic breast tumors, and in bone. By using genetic approaches, we find that bone-derived DKK1 contributes to the systemic DKK1 elevation in tumor-bearing female mice, while CAFs contribute to DKK1 at primary tumor site. Systemic and bone-specific DKK1 targeting reduce tumor growth. Intriguingly, deletion of CAF-derived DKK1 also limits breast cancer progression, without affecting its levels in circulation, and regardless of DKK1 expression in the tumor cells. While not directly supporting tumor proliferation, stromal-DKK1 suppresses NK cell activation and cytotoxicity by downregulating AKT/ERK/S6 phosphorylation. Importantly, increased DKK1 levels and reduced cytotoxic NK cells are detected in women with progressive breast cancer. Our findings indicate that DKK1 represents a barrier to anti-tumor immunity through suppression of NK cells.
© 2025. The Author(s).
-
Cancer Research
Preprint on BioRxiv : the Preprint Server for Biology on 6 December 2024 by Duguid, A., Malouf, C., et al.
Infant B-cell acute lymphoblastic leukaemia (B-ALL) is a rare, aggressive entity characterised by KMT2A rearrangements and poor outcomes. One of the unique features of KMT2A-rearranged infant ALL that contributes to these poor outcomes is a particularly high rate of central nervous system (CNS) involvement. There is a broad lack of understanding regarding the molecular processes and immune environment in CNS ALL. This hinders the development of targeted therapies for CNS ALL, which is increasingly essential given the potentially reduced efficacy of current immunotherapies in this specialised environment. In this study, we used an immune-competent KMT2A-AFF1+ infant B-ALL murine model to explore the cell-intrinsic and cell-extrinsic mechanisms that underpin this clinically significant complication. We show novel functional impacts on leukaemia propagating cells following exposure to the CNS niche which results in unique leukaemia repopulation dynamics. Transcriptomic and immune cell profiling by niche showed differences in the immune microenvironment between the CNS and bone marrow niches. The CNS niche demonstrates supressed T cell and macrophage activity which may be part of a wider CNS niche-specific immune escape mechanism. Our transcriptomic comparisons by niche also led us to explore the role of PI3K pathway activation in the propagation of leukaemia cells within the CNS niche. We identify miR-93 as a possible master regulator of this process. The importance of miR-93 is emphasised as it is shown to be upregulated in CNS leukaemia cells across multiple murine KMT2A-AFF1 B-ALL model systems and in primary KMT2A-AFF1 B-ALL patient samples. We conclude by showing impaired CNS engraftment of leukaemia cells upon miR-93 knockdown, cementing its importance in CNS leukaemia biology and opening up new CNS-specific therapeutic opportunities.
Preprint on Research Square on 5 December 2024 by Xian, S., Meng, F., et al.
Abstract Inflammatory bowel disease (IBD) is a relapsing disorder characterized by uncontrolled chronic inflammation of the gastrointestinal tract, posing a significant therapeutic challenge owing to the limited efficacy and undesirable side effects of current therapeutic options. A key pathological hallmark of IBD is the excessive production of reactive oxygen species (ROS). Hence, therapeutic strategies aimed at reducing ROS levels are promising for relieving these inflammatory conditions. Vitamin C—a natural nutrient for the human body—is well known for its potent antioxidant effects. However, the clinical development of vitamin C as a therapeutic drug has been hindered by its poor stability, rapid metabolism, and inadequate tissue accumulation. Herein, we report that the bioavailability of vitamin C can be enhanced by chemically reprogramming it with a small panel of long-chain fatty acids that aid in the aqueous self-assembly of the resulting drug conjugates to create self-deliverable nanoassemblies, enhancing their inflammation disease–oriented delivery and cellular uptake. In mice with dextran sulfate sodium–induced colitis, the optimal vitamin C–lipid nanoassemblies preferentially accumulated in inflamed colonic tissues following systemic administration and substantially ameliorated disease severity. We extended this strategy to incorporate the clinically approved glucocorticoid budesonide into the vitamin C nanosystem, facilitating a synergistic combination. In the chronic colitis model, the combination treatment reduced inflammation without compromising global immunity. Mechanistically, the treatment modulated the intestinal inflammatory microenvironment and altered the immune cell landscape, partly through regulation of the gut microbiome. Given its anticipated negligible side effects, this novel nanoassembly platform leveraging small-molecule lipidation may become a promising therapeutic for treating various inflammatory diseases.
Fungal symbiont transmitted by free-living mice promotes type 2 immunity.
In Nature on 1 December 2024 by Liao, Y., Gao, I. H., et al.
The gut mycobiota is crucial for intestinal homeostasis and immune function1. Yet its variability and inconsistent fungal colonization of laboratory mice hinders the study of the evolutionary and immune processes that underpin commensalism2,3. Here, we show that Kazachstania pintolopesii is a fungal commensal in wild urban and rural mice, with an exceptional ability to colonize the mouse gastrointestinal tract and dominate the gut mycobiome. Kazachstania pintolopesii colonization occurs in a bacteria-independent manner, results in enhanced colonization resistance to other fungi and is shielded from host immune surveillance, allowing commensal presence. Following changes in the mucosal environment, K. pintolopesii colonization triggers a type 2 immune response in mice and induces gastrointestinal eosinophilia. Mechanistically, we determined that K. pintolopesii activates type 2 immunity via the induction of epithelial IL-33 and downstream IL-33-ST2 signalling during mucus fluctuations. Kazachstania pintolopesii-induced type 2 immunity enhanced resistance to helminth infections or aggravated gastrointestinal allergy in a context-dependent manner. Our findings indicate that K. pintolopesii is a mouse commensal and serves as a valuable model organism for studying gut fungal commensalism and immunity in its native host. Its unnoticed presence in mouse facilities highlights the need to evaluate its influence on experimental outcomes and phenotypes.
© 2024. The Author(s), under exclusive licence to Springer Nature Limited.
-
Immunology and Microbiology
In Elife on 26 March 2021 by Sheng, J., Chen, Q., et al.
Fig.1.A

-
FC/FACS
-
Collected and cropped from Elife by CiteAb, provided under a CC-BY license
Image 1 of 3
In Elife on 26 March 2021 by Sheng, J., Chen, Q., et al.
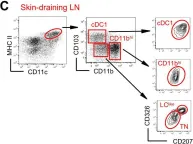
Fig.1.C

-
FC/FACS
-
Collected and cropped from Elife by CiteAb, provided under a CC-BY license
Image 1 of 3
In Elife on 26 March 2021 by Sheng, J., Chen, Q., et al.
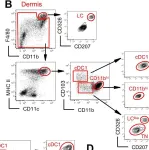
Fig.1.B

-
FC/FACS
-
Collected and cropped from Elife by CiteAb, provided under a CC-BY license
Image 1 of 3