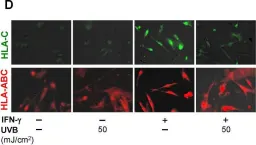
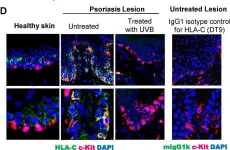
UVB phototherapy effectively treats psoriasis. Although it suppresses both innate and adaptive immunity, it remains unclear why UVB irradiation is primarily effective for T-cell-mediated but not inflammatory skin diseases of other etiologies. Using a Vα3S1/Vβ13S1 T-cell receptor (TCR) from a lesional psoriatic CD8+ T-cell clone, we recently demonstrated that in psoriasis, the major psoriasis risk allele HLA-C*06:02 mediates an autoimmune response of CD8+ T-cells against melanocytes by presenting a melanocyte autoantigen. We now investigate the effect of UVB irradiation on melanocyte immunogenicity using the psoriatic Vα3S1/Vβ13S1 TCR in a reporter assay. The immunogenicity of melanocytes for the Vα3S1/Vβ13S1 TCR depended on the up-regulation of HLA-C expression by IFN-γ. UVB irradiation reduced the stimulatory capacity of IFN-γ-conditioned melanocytes for the Vα3S1/Vβ13S1 TCR by suppressing key IFN-γ-induced MHC-class I transcriptional regulators (STAT1, IRF1, NLRC5), the HLA-C-specific transcription factor Oct1, and by inducing miR-148a, which specifically inhibits HLA-C expression. This resulted in the suppression of the IFN-γ-induced expression of HLA-class I molecules and, in particular, an almost complete loss of HLA-C expression. We conclude that suppression of the inflammatory increase in HLA-class I expression and antigen-presentation may contribute to the efficacy of UVB phototherapy in T-cell-mediated skin diseases. The pronounced downregulation of HLA-C on melanocytes could render psoriasis, as HLA-C-associated disease, particularly susceptible to this effect.
Product Citations: 11
In International Journal of Molecular Sciences on 21 March 2025 by Arakawa, Y., Arakawa, A., et al.
-
ICC-IF
-
IHC-IF
Cancer cells suppress NK activity by actin-driven polarisation of inhibitory ligands at the synapse
Preprint on BioRxiv : the Preprint Server for Biology on 10 February 2025 by Hoffmann, C., Filali, L., et al.
Natural killer (NK) cells engage target cells via the immunological synapse, where inhibitory and activating signals determine whether NK cell cytotoxicity is suppressed or activated. We report that cancer cells can rapidly remodel their actin cytoskeleton upon NK cell engagement, leading to F-actin accumulation at the synapse. This process inhibits NK cell activation as indicated by impaired MTOC and lytic granule polarization. Exploring the underlying mechanism, we found that actin remodelling drives the recruitment of inhibitory ligands, such as HLA-A, -B, and -C, to the synapse. Disrupting HLA interaction with their cognate inhibitory receptors KIRs restored NK cell activation. Using NK cells expressing inhibitory KIR receptors, matched or unmatched to HLA molecules on cancer cells, we show that synaptic F-actin accumulation and matching KIR-HLA interactions jointly suppress NK cell cytotoxicity. Our findings reveal a novel immune evasion strategy in which cancer cells impair NK cell activation by altering synaptic signalling through actin cytoskeleton-driven recruitment of inhibitory signals to the immunological synapse.
-
ICC-IF
-
Cancer Research
-
Cell Biology
-
Neuroscience
In Redox Biology on 1 November 2024 by Heirman, P., Verswyvel, H., et al.
Non-thermal plasma (NTP) shows promise as a potent anti-cancer therapy with both cytotoxic and immunomodulatory effects. In this study, we investigate the chemical and biological effects of NTP-induced oxidation on several key, determinant immune checkpoints of natural killer (NK) cell function. We used molecular dynamics (MD) and umbrella sampling simulations to investigate the effect of NTP-induced oxidative changes on the MHC-I complexes HLA-Cw4 and HLA-E. Our simulations indicate that these chemical alterations do not significantly affect the binding affinity of these markers to their corresponding NK cell receptor, which is supported with experimental read-outs of ligand expression on human head and neck squamous cell carcinoma cells after NTP application. Broadening our scope to other key ligands for NK cell reactivity, we demonstrate rapid reduction in CD155 and CD112, target ligands of the inhibitory TIGIT axis, and in immune checkpoint CD73 immediately after treatment. Besides these transient chemical alterations, the reactive species in NTP cause a cascade of downstream cellular reactions. This is underlined by the upregulation of the stress proteins MICA/B, potent ligands for NK cell activation, 24 h post treatment. Taken together, this work corroborates the immunomodulatory potential of NTP, and sheds light on the interaction mechanisms between NTP and cancer cells.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
-
Immunology and Microbiology
In Cytotherapy on 1 July 2023 by Feng, C., Zhang, T., et al.
Recently, immune escape has been considered as a factor leading to relapse of acute myeloid leukemia (AML). In our previous study, heme oxygenase 1 (HO-1) proved to play an essential role in the proliferation and drug resistance of AML cells. In addition, recent studies by our group have shown that HO-1 is involved in immune escape in AML. Nevertheless, the specific mechanism by which HO-1 mediates immune escape in AML remains unclear.
In this study, we found that patients with AML and an overexpression of HO-1 had a high rate of recurrence. In vitro, overexpression of HO-1 attenuated the toxicity of natural killer (NK) cells to AML cells. Further study indicated that HO-1 overexpression inhibited human leukocyte antigen-C and reduced the cytotoxicity of NK cells to AML cells, leading to AML relapse. Mechanistically, HO-1 inhibited human leukocyte antigen-C expression by activating the JNK/C-Jun signaling pathway.
In AML, HO-1 inhibits cytotoxicity of NK cells by inhibiting the expression of HLA-C, thus causing immune escape of AML cells.
NK cell-mediated innate immunity is important for the fight against tumors, especially when acquired immunity is depleted and dysfunctional, and the HO-1/HLA-C axis can induce functional changes in NK cells in AML. Anti-HO-1 treatment can promote the antitumor effect of NK cells and may play an important role in the treatment of AML.
Copyright © 2023 International Society for Cell & Gene Therapy. Published by Elsevier Inc. All rights reserved.
-
Cancer Research
-
Immunology and Microbiology
In STAR Protocols on 11 May 2023 by Hamilton, I., Ikumi, N. M., et al.
Fetal extravillous trophoblasts (EVTs) are the most invasive cells of the placenta and play a key role in modulating maternal immune responses. Here, we present a protocol to purify and culture human leukocyte antigen-G (HLA-G)+ EVTs. We describe steps for tissue dissection, tissue digestion, density gradient centrifugation, and cell sorting, and we provide detailed methods to determine EVT function. HLA-G+ EVTs are isolated from two maternal-fetal interfaces: the chorionic membrane and the basalis/villous tissue. This protocol allows in-depth functional investigation of maternal immune interactions with HLA-G+ EVTs. For complete details on the use and execution of this protocol, please refer to Papuchova et al. (2020),1 Salvany-Celades et al. (2019),2 Tilburgs et al. (2015),3 Tilburgs et al. (2015),4 van der Zwan et al. (2018).5.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
In Int J Mol Sci on 21 March 2025 by Arakawa, Y., Arakawa, A., et al.
Fig.1.D

-
ICC-IF
-
Collected and cropped from Int J Mol Sci by CiteAb, provided under a CC-BY license
Image 1 of 4
In Int J Mol Sci on 21 March 2025 by Arakawa, Y., Arakawa, A., et al.
Fig.4.D

-
IHC-IF
-
Collected and cropped from Int J Mol Sci by CiteAb, provided under a CC-BY license
Image 1 of 4
In Cancers (Basel) on 13 September 2020 by Tao, L., Farooq, M. A., et al.
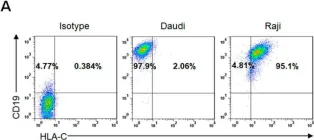
Fig.2.A

-
FC/FACS
-
Collected and cropped from Cancers (Basel) by CiteAb, provided under a CC-BY license
Image 1 of 4
In Cancers (Basel) on 13 September 2020 by Tao, L., Farooq, M. A., et al.
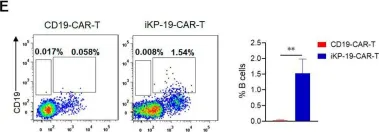
Fig.6.E

-
FC/FACS
-
Collected and cropped from Cancers (Basel) by CiteAb, provided under a CC-BY license
Image 1 of 4