Notochordal cells (NCs) present in the nucleus pulposus (NP) of the developing human intervertebral disc (IVD) disappear during the first decade of life. This loss coincides with the onset of IVD degeneration, therefore these cells are hypothesized to be important in NP homeostasis. Putative NC-derived (CD24+) and progenitor (TIE2+/GD2+) cell sub-populations have previously been identified in the adult human NP, but their characteristics have yet to be compared. Here, we used CD24, TIE2 and GD2 to identify and then isolate discrete cell sub-populations to assess cell phenotype.
CD24, GD2 and TIE2 positivity was assessed in a cohort of human pediatric and adult NP samples across a range of ages and histological degeneration grades using immunohistochemistry and flow cytometry. FACS sorting was used to isolate different cell sub-populations (CD24+/GD2+; CD24+/GD2-; CD24-/GD2+; CD24-/GD2-). Cell phenotype was assessed using qPCR for known NC and NP markers as well as catabolic genes.
CD24+ and GD2+ cells were localized in all samples, irrespective of age or degeneration grade, while TIE2+ cell number was consistently very low. The same positivity trend was confirmed using flow cytometry. A small CD24+/GD2+ sub-population was present and maintained marker expression with time in culture. CD24+ subpopulations showed a significantly higher expression of NC markers than the CD24- subpopulations and unsorted samples, suggesting a healthier phenotype in the CD24+ cells. GD2 did not appear to influence gene expression.
This study provides a better understanding of different cell sub-populations present in the adult NP, with identification of CD24+/GD2+ cells that are maintained with aging and degeneration. Healthy, NC-like phenotypic profiles appeared reliant on CD24, rather than GD2. The study highlights the importance of studying discrete cell sub-populations, especially CD24+ NP cells to better understand their role in NP homeostasis.
© 2024 The Author(s). JOR Spine published by Wiley Periodicals LLC on behalf of Orthopaedic Research Society.
Product Citations: 3
In JOR Spine on 1 December 2024 by Ionescu, A. M., Baird, P., et al.
-
Homo sapiens (Human)
In Biochemical Pharmacology on 1 August 2022 by Thirugnanam, K., Prabhudesai, S., et al.
In the developing vasculature, cilia, microtubule-based organelles that project from the apical surface of endothelial cells (ECs), have been identified to function cell autonomously to promote vascular integrity and prevent hemorrhage. To date, the underlying mechanisms of endothelial cilia formation (ciliogenesis) are not fully understood. Understanding these mechanisms is likely to open new avenues for targeting EC-cilia to promote vascular stability. Here, we hypothesized that brain ECs ciliogenesis and the underlying mechanisms that control this process are critical for brain vascular stability. To investigate this hypothesis, we utilized multiple approaches including developmental zebrafish model system and primary cell culture systems. In the p21 activated kinase 2 (pak2a) zebrafish vascular stability mutant [redhead (rhd)] that shows cerebral hemorrhage, we observed significant decrease in cilia-inducing protein ADP Ribosylation Factor Like GTPase 13B (Arl13b), and a 4-fold decrease in cilia numbers. Overexpressing ARL13B-GFP fusion mRNA rescues the cilia numbers (1-2-fold) in brain vessels, and the cerebral hemorrhage phenotype. Further, this phenotypic rescue occurs at a critical time in development (24 h post fertilization), prior to initiation of blood flow to the brain vessels. Extensive biochemical mechanistic studies in primary human brain microvascular ECs implicate ligands platelet-derived growth factor-BB (PDGF-BB), and vascular endothelial growth factor-A (VEGF-A) trigger PAK2-ARL13B ciliogenesis and signal through cell surface VEGFR-2 receptor. Thus, collectively, we have implicated a critical brain ECs ciliogenesis signal that converges on PAK2-ARL13B proteins to promote vascular stability.
Copyright © 2022 Elsevier Inc. All rights reserved.
-
Biochemistry and Molecular biology
In BMC Cell Biology on 6 March 2007 by Nguyen, V. P., Chen, S. H., et al.
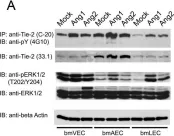
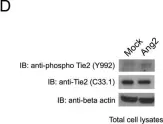
The lymphatic system complements the blood circulatory system in absorption and transport of nutrients, and in the maintenance of homeostasis. Angiopoietins 1 and 2 (Ang1 and Ang2) are regulators of both angiogenesis and lymphangiogenesis through the Tek/Tie-2 receptor tyrosine kinase. The response of endothelial cells to stimulation with either Ang1 or Ang2 is thought to be dependent upon the origin of the endothelial cells. In this study, we examined the effects of the angiopoietins on lymphatic, venous and arterial primary endothelial cells (bmLEC, bmVEC and bmAEC, respectively), which were isolated and cultured from bovine mesenteric vessels.
BmLEC, bmVEC and bmAEC cell populations all express Tie-2 and were shown to express the appropriate cellular markers Prox-1, VEGFR3, and Neuropilin-1 that define the particular origin of each preparation. We showed that while bmLECs responded slightly more readily to angiopoietin-2 (Ang2) stimulation, bmVECs and bmAECs were more sensitive to Ang1 stimulation. Furthermore, exposure of bmLECs to Ang2 induced marginally higher levels of proliferation and survival than did exposure to Ang1. However, exposure to Ang1 resulted in higher levels of migration in bmLECs than did to Ang2.
Our results suggest that although both Ang1 and Ang2 can activate the Tie-2 receptor in bmLECs, Ang1 and Ang2 may have distinct roles in mesenteric lymphatic endothelial cells.
-
WB
-
Cardiovascular biology
-
Cell Biology
In BMC Cell Biol on 6 March 2007 by Nguyen, V. P., Chen, S. H., et al.
Fig.3.A

-
WB
-
Collected and cropped from BMC Cell Biol by CiteAb, provided under a CC-BY license
Image 1 of 2
In BMC Cell Biol on 6 March 2007 by Nguyen, V. P., Chen, S. H., et al.
Fig.3.D

-
WB
-
Collected and cropped from BMC Cell Biol by CiteAb, provided under a CC-BY license
Image 1 of 2