The expansion of the neocortex is a hallmark of human evolution and is closely linked to neural stem cell biology. Yet, the epigenetic mechanisms driving divergent gene regulation during primate neurogenesis remain elusive. Here, we comprehensively mapped 3D genome organization, chromatin accessibility and gene expression in induced pluripotent stem cells and derived neural stem cells from human, chimpanzee, gorilla and macaque. We identified human-specific epigenetic signatures including cis-regulatory regions and enhancer-promoter interactions and linked them to gene regulatory dynamics. Deep learning models revealed that complex regulatory grammar at cis-regulatory regions, including transcription factor binding sites, local context and higher-order chromatin organization, underlies species and cell type-specific differences. High-resolution Hi-C uncovered unexpected global shifts in 3D genome architecture in chimpanzee and gorilla neural stem cells while topologically associating domains remain remarkably conserved. Notably, species-specific genes interacted with multiple differentially accessible regions, suggesting that synergistic enhancer activation is a key mechanism driving epigenome evolution. These findings provide new insights into the epigenetic basis of primate brain evolution.
Product Citations: 9
3D Epigenome Evolution Underlies Divergent Gene Regulatory Programs in Primate Neural Development
Preprint on BioRxiv : the Preprint Server for Biology on 12 March 2025 by Vangelisti, S., Chong, F., et al.
Preprint on BioRxiv : the Preprint Server for Biology on 2 October 2024 by Ditzer, N., Şenoğlu, E., et al.
Summary Epigenetic mechanisms regulate gene expression programs during neurogenesis, but the extent of epigenetic remodelling during human cortical development remains unknown. Here, we characterize the epigenetic landscape of the human developing neocortex by leveraging Epi-CyTOF, a mass cytometry-based approach for the simultaneous single cell analysis of more than 30 epigenetic marks. We identify H3K27me3, deposited by Polycomb Repressive Complex 2 (PRC2), as the modification with the strongest cell type-specific enrichment. Inhibition of PRC2 in human cortical organoids resulted in a shift of neural progenitor cell (NPC) proliferation towards differentiation. Cell type- specific profiling of H3K27me3 not only identified neuronal differentiation genes in the human neocortex, but also extra-cellular matrix (ECM) genes. PRC2 inhibition resulted in increased production of the proteoglycan Syndecan 1. Overall, this study comprehensively characterizes the epigenetic state of specific neural cell types and highlights a novel role for H3K27me3 in regulating the ECM composition in the human developing neocortex.
-
Homo sapiens (Human)
In International Journal of Molecular Sciences on 26 September 2024 by Repas, J., Frlic, T., et al.
2-deoxy-D-glucose (2DG) is a glycolysis and protein N-glycosylation inhibitor with promising anti-tumor and immunomodulatory effects. However, 2DG can also suppress T cell function, including IFN-γ secretion. Few human T cell studies have studied low-dose 2DG, which can increase IFN-γ in a Jurkat clone. We therefore investigated 2DG's effect on IFN-γ in activated human T cells from PBMCs, with 2DG treatment commenced either concurrently with activation or 48 h after activation. Concurrent 2DG treatment decreased IFN-γ secretion in a dose-dependent manner. However, 2DG treatment of pre-activated T cells had a hormetic effect on IFN-γ, with 0.15-0.6 mM 2DG (achievable in vivo) increasing and >2.4 mM 2DG reducing its secretion. In contrast, IL-2 levels declined monotonously with increasing 2DG concentration. Lower 2DG concentrations reduced PD-1 and increased CD69 expression regardless of treatment timing. The absence of increased T-bet or Eomes expression or IFNG transcription suggests another downstream mechanism. 2DG dose-dependently induced the unfolded protein response, suggesting a possible role in increased IFN-γ secretion, possibly by increasing the ER folding capacity for IFN-γ via increased chaperone expression. Overall, low-dose, short-term 2DG exposure could potentially improve the T cell anti-tumor response.
-
Immunology and Microbiology
In Frontiers in Endocrinology on 20 December 2023 by Repas, J., Peternel, L., et al.
Modulation of immune cell metabolism is one of promising strategies to improve cancer immunotherapies. Metformin is an anti-diabetic drug with potential anti-cancer effects, ranging from normalization of blood glucose and insulin levels, direct anti-proliferative effects on cancer cells to emerging immunomodulatory effects on anti-tumor immunity. Metformin can reduce tumor hypoxia and PD-L1 expression, as well as normalize or improve T cell function and potentiate the effect of immune checkpoint inhibitors, making it a promising adjuvant to immunotherapy of tumors with poor response such as triple negative breast cancer (TNBC). However, although the effects of metformin on cancer cells are glucose-dependent, the role of glucose in modulating its effect on T cells has not been systematically studied. We thus investigated the effect of metformin as a function of glucose level on Jurkat cell and PBMC T cell models in vitro. While low metformin concentrations had little effect on T cell function, high concentration reduced proliferation and IFN-γ secretion in both models and induced a shift in T cell populations from memory to effector subsets. The PD-1/CD69 ratio was improved by high metformin in T cells from PBMC. Low glucose and metformin synergistically reduced PD-1 and CD69 expression and IFN-γ secretion in T cells from PBMC. Low glucose level itself suppressed Jurkat cell function due to their limited metabolic plasticity, but had limited effects on T cells from PBMC apart from reduced proliferation. Conversely, high glucose did not strongly affect either T cell model. Metformin in combination with glycolysis inhibitor 2-deoxy-D-glucose (2DG) reduced PD-1 in Jurkat cells, but also strongly suppressed their function. However, low, physiologically achievable 2DG concentration itself reduced PD-1 while mostly maintaining IL-2 secretion and, interestingly, even strongly increased IFN-γ secretion regardless of glucose level. Overall, glucose metabolism can importantly influence some of the effects of metformin on T cell functionality in the tumor microenvironment. Additionally, we show that 2DG could potentially improve the anti-tumor T cell response.
Copyright © 2023 Repas, Peternel, Sourij and Pavlin.
-
Endocrinology and Physiology
-
Immunology and Microbiology
In Nature Immunology on 1 April 2023 by Lo, W. L., Kuhlmann, M., et al.
Mature T cells must discriminate between brief interactions with self-peptides and prolonged binding to agonists. The kinetic proofreading model posits that certain T-cell antigen receptor signaling nodes serve as molecular timers to facilitate such discrimination. However, the physiological significance of this regulatory mechanism and the pathological consequences of disrupting it are unknown. Here we report that accelerating the normally slow phosphorylation of the linker for activation of T cells (LAT) residue Y136 by introducing an adjacent Gly135Asp alteration (LATG135D) disrupts ligand discrimination in vivo. The enhanced self-reactivity of LATG135D T cells triggers excessive thymic negative selection and promotes T-cell anergy. During Listeria infection, LATG135D T cells expand more than wild-type counterparts in response to very weak stimuli but display an imbalance between effector and memory responses. Moreover, despite their enhanced engagement of central and peripheral tolerance mechanisms, mice bearing LATG135D show features associated with autoimmunity and immunopathology. Our data reveal the importance of kinetic proofreading in balancing tolerance and immunity.
© 2023. The Author(s).
-
Mus musculus (House mouse)
-
Immunology and Microbiology
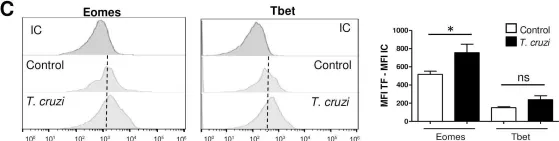
In PLoS Pathog on 1 January 2019 by Baez, N. S., Cerbán, F., et al.
Fig.2.C

-
FC/FACS
-
Collected and cropped from PLoS Pathog by CiteAb, provided under a CC-BY license
Image 1 of 1