Osteosarcoma (OS) with pulmonary metastasis remains challenging due to limited treatment options and the immunosuppressive nature of the tumor microenvironment (TME). Bacteria-mediated cancer therapy has emerged as a promising strategy for solid tumors but often suffers from limited efficacy due to the immunosuppressive TME, which restricts the intensity and durability of the antitumor immune response. To overcome these challenges, we engineered a novel Salmonella strain, VNP20009-CCL2-CXCL9 (VNP-C-C), leveraging the intrinsic tumor tropism of Salmonella typhimurium VNP20009 (VNP) and improving immune modulation through the recruitment of effector immune cells into the TME by the chemokines CCL2 and CXCL9.
VNP-C-C was genetically engineered through electroporation of Plac-CCL2-CXCL9 plasmid and validated in vitro. Its antitumor efficacy, immune regulation capacity and immunomodulatory mechanisms were evaluated in vitro by using OS cell lines and immune cells (dendritic cells (DCs) and macrophages (Mφs)) and in vivo by using both immunocompromised and immunocompetent mouse models of OS lung metastasis.
VNP-C-C effectively accumulated within tumors, triggering immunogenic cell death and subsequently activating the cyclic GMP-AMP synthase (cGAS)/stimulator of interferon genes (STING) pathway, thereby robustly promoting type I interferon secretion. The chemokines CCL2 and CXCL9 amplified the immune response by recruiting DCs, Mφs, and T cells to the TME. This orchestrated immune modulation reprogrammed tumor-associated macrophages to an antitumor phenotype, induced DCs maturation, significantly increased T-cell infiltration and activation within tumors, and promoted systemic T-cell memory formation in peripheral lymphoid organs. These effects collectively inhibited OS lung metastasis progression and provided survival benefits in mouse models.
The engineered bacterial strain VNP-C-C effectively converts the OS lung metastatic TME into a pro-inflammatory milieu, thereby stimulating robust innate and adaptive immune responses. This offers a highly promising therapeutic avenue for OS lung metastasis with considerable translational potential in cancer immunotherapy.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Product Citations: 21
In Journal for Immunotherapy of Cancer on 1 July 2025 by Liu, R., Liu, Q., et al.
-
FC/FACS
-
Mus musculus (House mouse)
-
Cancer Research
-
Immunology and Microbiology
In Journal of Clinical Medicine on 7 March 2025 by Byun, J. E., Lee, J. W., et al.
Background/Objectives: Regulation of acute inflammatory responses is crucial for host mortality and morbidity induced by pathogens. The pathogenesis of acute respiratory distress syndrome (ARDS) and sepsis are associated with systemic inflammation. p38 MAPK is a crucial regulator of inflammatory responses and is a potential target for acute inflammatory diseases, including ARDS and sepsis. We investigated the therapeutic effects of the TAT-TN13 peptide (TN13) on severe inflammatory diseases, including ARDS and sepsis, in vivo. Methods: To establish the ARDS model, C57BL/6 mice were intranasally (i.n.) administered lipopolysaccharide (LPS; 5 mg/kg, 40 µL) to induce lung inflammation. As a positive control, dexamethasone (DEX; 0.2 mg/kg) was administered intraperitoneally (i.n.) 1 h post-LPS exposure. In the experimental groups, TN13 was administered intranasally (i.n.) at doses of 2.5 mg or 5 mg/kg at the same time point. In the LPS-induced sepsis model, mice received an intraperitoneal injection of LPS (20 mg/kg) to induce systemic inflammation. TN13 (25 mg/kg, i.p.) was administered 1 h after LPS treatment. Control mice received phosphate-buffered saline (PBS). Lung histopathology, inflammatory cell infiltration, cytokine levels, and survival rates were assessed to evaluate TN13 efficacy. Results: TN13 significantly reduced inflammatory cell recruitment and cytokine production in the lungs, thereby mitigating LPS-induced ARDS. In the sepsis model, TN13 treatment improved survival rates by suppressing inflammatory responses. Mechanistically, TN13 exerted its effects by inhibiting the p38 MAPK/NF-κB signaling pathway. Conclusions: These results collectively suggested that TN13 could be an effective treatment option for severe inflammatory diseases.
In Redox Biology on 1 March 2025 by Zhang, Q., Liu, X., et al.
Chemotherapy is important in the systemic therapy for breast cancer. However, after chemotherapy, the left living tumour cells are more progressive. There is an urgent need to study the underlying mechanism which is still unclear to further improve the therapeutic efficacy of chemotherapy in breast cancer. Here we find a pro-tumour effect of the apoptotic cells induced by the chemotherapy, which is mediated by a new subset of macrophages undergoing LC3-associated phagocytosis (LAP). By transferring exosomal S100A11 into the living tumour cells after chemotherapy, the macrophage exhibits a more pro-tumour phenotype than classic M2-type macrophages. Moreover, S100A11 binds to IFITM3, inducing Akt phosphorylation of living tumour cells after chemotherapy, which promotes tumour progression. Of note, Akt inhibitor can enhance the therapeutic effcicay of chemotherapy in breast cancer. This study provides a novel mechanistic link between tumour-associated macrophages and breast cancer, uncovering Akt as a potential therapeutic target to improve chemotherapy efficacy.
Copyright © 2025 The Authors. Published by Elsevier B.V. All rights reserved.
-
Mus musculus (House mouse)
-
Cancer Research
In Journal of Nanobiotechnology on 23 December 2024 by Liu, F., Wang, X., et al.
The multi-biological barriers present in the inflammatory microenvironment severely limit the targeted aggregation of anti-inflammatory drugs in the lesion area. However, conventional responsive drug carriers inevitably come into contact with several pro-responsive stimulatory mediators simultaneously, leading to premature drug release and loss of most therapeutic effects. Breaking through the multi-level barriers of the inflammatory microenvironment is essential to improve the enrichment and bioavailability of drugs.
In this study, we propose a novel two-stage structural strategy to build shielded cascades of targeted nanocarriers (FA-PTP@Que) through inflammatory mediators, using cascade structures to cross multiple environmental barriers. The cascade structure of FA-PTP@Que is responsive to inflammatory mediators and exhibits ideal pathological microenvironmental response and drug release properties. FA-PTP@Que has shown good macrophage regulation and anti-inflammatory effects by efficiently targeting macrophages, scavenging intracellular reactive oxygen species (ROS), and down-regulating the secretion of pro-inflammatory factors. Significantly, in mice with arthritis and colitis, FA-PTP@Que enriches and targets macrophages at the sites of arthritis and colitis, showing significant anti-inflammatory effects.
FA-PTP@Que combines active chemotaxis of nanocarriers to inflammatory tissues and active targeting of effector cells, acting precisely at each barrier level in different microenvironments by responding to inflammatory mediators and overcoming the multiple barriers in the inflammatory microenvironment. This innovative strategy can effectively break through various inflammatory microenvironments and has the potential application to other inflammatory diseases.
© 2024. The Author(s).
-
FC/FACS
-
Mus musculus (House mouse)
-
Immunology and Microbiology
In Journal of Nanobiotechnology on 19 December 2024 by Lan, H., Zhou, Z., et al.
Sepsis is a severe immune response to pathogens that is associated with high mortality rate and a paucity of efficacious treatment options. It is characterized by the hyperactivation of macrophages and the occurrence of cytokine storms. Given the anti-inflammatory properties of M2 macrophages and their derived apoptotic bodies (AB), as well as the specific uptake of these by macrophages, a novel approach was employed to combine AB with artificial liposomes to create apoptotic body based biomimetic hybrid nanovesicles (L-AB). The L-AB effectively inherited "eat me" signaling molecules on the surface of the AB, thereby facilitating their targeted uptake by macrophages in both in vitro and in vivo settings. The administration of L-AB for the delivery of dexamethasone effectively augmented the therapeutic efficacy of the drug, mitigated macrophage hyperactivation and tissue damage in vivo, and consequently enhanced the survival rate of septic mice. Taken together, these findings suggest that the apoptotic body biomimetic nanovesicles may represent a potential drug delivery system capable of specifically targeting macrophages for the treatment of sepsis.
© 2024. The Author(s).
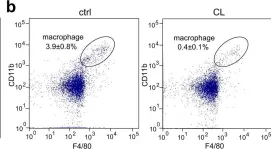
In NPJ Regen Med on 2 May 2023 by Zhou, Y., Ye, Z., et al.
Fig.1.B

-
FC/FACS
-
Collected and cropped from NPJ Regen Med by CiteAb, provided under a CC-BY license
Image 1 of 1