Senescence-associated secretory phenotype (SASP) mediates the biological effects of senescent cells on the tissue microenvironment and contributes to ageing-associated disease progression. ACSS2 produces acetyl-CoA from acetate and epigenetically controls gene expression through histone acetylation under various circumstances. However, whether and how ACSS2 regulates cellular senescence remains unclear. Here, we show that pharmacological inhibition and deletion of Acss2 in mice blunts SASP and abrogates the pro-tumorigenic and immune surveillance functions of senescent cells. Mechanistically, ACSS2 directly interacts with and promotes the acetylation of PAICS, a key enzyme for purine biosynthesis. The acetylation of PAICS promotes autophagy-mediated degradation of PAICS to limit purine metabolism and reduces dNTP pools for DNA repair, exacerbating cytoplasmic chromatin fragment accumulation and SASP. Altogether, our work links ACSS2-mediated local acetyl-CoA generation to purine metabolism through PAICS acetylation that dictates the functionality of SASP, and identifies ACSS2 as a potential senomorphic target to prevent senescence-associated diseases.
© 2025. The Author(s).
Product Citations: 69
In Nature Communications on 28 February 2025 by Yang, L., You, J., et al.
-
WB
-
Homo sapiens (Human)
-
Genetics
Preprint on BioRxiv : the Preprint Server for Biology on 26 September 2024 by Ai, Y., Ding, Q., et al.
SUMMARY Alternative polyadenylation (APA) generates mRNA isoforms with different lengths of the 3’ untranslated region (3’ UTR). The tissue inhibitor of metalloproteinase 2 (TIMP2) plays a key role in extracellular matrix remodeling under various developmental and disease conditions. Both human and mouse genes encoding TIMP2 contain two highly conserved 3’UTR APA sites, leading to mRNA isoforms that differ substantially in 3’UTR size. APA of Timp2 is one of the most significantly regulated events in multiple cell differentiation lineages. Here we show that Timp2 APA is highly regulated in transformation of NIH3T3 cells by the oncogene HRAS G12V . Perturbations of isoform expression with long 3’UTR isoform-specific knockdown or genomic removal of the alternative UTR (aUTR) region indicate that the long 3’UTR isoform contributes to the secreted Timp2 protein much more than the short 3’UTR isoform. The short and long 3’UTR isoforms differ in subcellular localization to endoplasmic reticulum (ER). Strikingly, Timp2 aUTR enhances secreted protein expression but no effect on intracellular proteins in reporter assays. Furthermore, downregulation of Timp2 long isoform mitigates gene expression changes elicited by HRAS G12V . Together, our data indicate that regulation of Timp2 protein expression through APA isoform changes is an integral part of RAS-mediated cell transformation and 3’UTR isoforms of Timp2 can have distinct impacts on expression of secreted vs. intracellular proteins.
In Nature Communications on 26 June 2024 by Wang, C., Tanizawa, H., et al.
METTL3 is the catalytic subunit of the methyltransferase complex, which mediates m6A modification to regulate gene expression. In addition, METTL3 regulates transcription in an enzymatic activity-independent manner by driving changes in high-order chromatin structure. However, how these functions of the methyltransferase complex are coordinated remains unknown. Here we show that the methyltransferase complex coordinates its enzymatic activity-dependent and independent functions to regulate cellular senescence, a state of stable cell growth arrest. Specifically, METTL3-mediated chromatin loops induce Hexokinase 2 expression through the three-dimensional chromatin organization during senescence. Elevated Hexokinase 2 expression subsequently promotes liquid-liquid phase separation, manifesting as stress granule phase separation, by driving metabolic reprogramming. This correlates with an impairment of translation of cell-cycle related mRNAs harboring polymethylated m6A sites. In summary, our results report a coordination of m6A-dependent and -independent function of the methyltransferase complex in regulating senescence through phase separation driven by metabolic reprogramming.
© 2024. The Author(s).
-
Biochemistry and Molecular biology
-
Cell Biology
In Physiological Reports on 1 March 2024 by Uda, M., Yoshihara, T., et al.
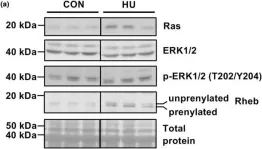
Fast-twitch muscles are less susceptible to disuse atrophy, activate the mechanistic target of rapamycin complex 1 (mTORC1) signaling pathway, and increase protein synthesis under prolonged muscle disuse conditions. However, the mechanism underlying prolonged muscle disuse-induced mTORC1 signaling activation remains unclear. The mevalonate pathway activates the mTORC1 signaling pathway via the prenylation and activation of Ras homolog enriched in brain (Rheb). Therefore, we investigated the effects of hindlimb unloading (HU) for 14 days on the mevalonate and mTORC1 signaling pathways in the plantaris muscle, a fast-twitch muscle, in adult male rats. Rats were divided into HU and control groups. The plantaris muscles of both groups were harvested after the treatment period, and the expression and phosphorylation levels of metabolic and intracellular signaling proteins were analyzed using Western blotting. We found that HU increased the expression of 3-hydroxy-3-methylglutaryl-coenzyme A reductase, the rate-limiting enzyme of the mevalonate pathway, and activated the mTORC1 signaling pathway without activating AKT, an upstream activator of mTORC1. Furthermore, HU increased prenylated Rheb. Collectively, these findings suggest that the activated mevalonate pathway may be involved in the activation of the Rheb/mTORC1 signaling pathway without AKT activation in fast-twitch muscles under prolonged disuse conditions.
© 2024 The Authors. Physiological Reports published by Wiley Periodicals LLC on behalf of The Physiological Society and the American Physiological Society.
-
WB
-
Rattus norvegicus (Rat)
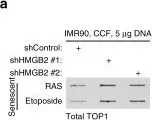
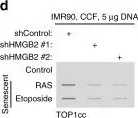
In Nature Aging on 1 February 2024 by Hao, X., Zhao, B., et al.
Sterile inflammation, also known as 'inflammaging', is a hallmark of tissue aging. Cellular senescence contributes to tissue aging, in part, through the secretion of proinflammatory factors collectively known as the senescence-associated secretory phenotype (SASP). The genetic variability of thioredoxin reductase 1 (TXNRD1) is associated with aging and age-associated phenotypes such as late-life survival, activity of daily living and physical performance in old age. TXNRD1's role in regulating tissue aging has been attributed to its enzymatic role in cellular redox regulation. Here, we show that TXNRD1 drives the SASP and inflammaging through the cyclic GMP-AMP synthase (cGAS)-stimulator of interferon genes (STING) innate immune response pathway independently of its enzymatic activity. TXNRD1 localizes to cytoplasmic chromatin fragments and interacts with cGAS in a senescence-status-dependent manner, which is necessary for the SASP. TXNRD1 enhances the enzymatic activity of cGAS. TXNRD1 is required for both the tumor-promoting and immune surveillance functions of senescent cells, which are mediated by the SASP in vivo in mouse models. Treatment of aged mice with a TXNRD1 inhibitor that disrupts its interaction with cGAS, but not with an inhibitor of its enzymatic activity alone, downregulated markers of inflammaging in several tissues. In summary, our results show that TXNRD1 promotes the SASP through the innate immune response, with implications for inflammaging. This suggests that the TXNRD1-cGAS interaction is a relevant target for selectively suppressing inflammaging.
© 2024. The Author(s), under exclusive licence to Springer Nature America, Inc.
-
Immunology and Microbiology
In Physiol Rep on 1 March 2024 by Uda, M., Yoshihara, T., et al.
Fig.7.A

-
WB
-
Rattus norvegicus (Rat)
Collected and cropped from Physiol Rep by CiteAb, provided under a CC-BY license
Image 1 of 15
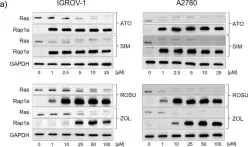
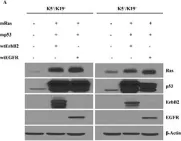
In BMC Cancer on 29 July 2020 by Göbel, A., Zinna, V. M., et al.
Fig.1.A

-
WB
-
Homo sapiens (Human)
Collected and cropped from BMC Cancer by CiteAb, provided under a CC-BY license
Image 1 of 15
In Nat Commun on 19 February 2020 by Zhao, B., Liu, P., et al.
Fig.2.A

-
WB
-
Homo sapiens (Human)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 15
In Nat Commun on 19 February 2020 by Zhao, B., Liu, P., et al.
Fig.2.D

-
WB
-
Homo sapiens (Human)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 15
In Sci Rep on 23 October 2018 by Mohamed, A. D., Shah, N., et al.
Fig.2.A

-
WB
-
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 15
In Genome Biol on 25 July 2016 by Nelson, D. M., Jaber-Hijazi, F., et al.
Fig.1.C

-
WB
-
Collected and cropped from Genome Biol by CiteAb, provided under a CC-BY license
Image 1 of 15
In Genome Biol on 25 July 2016 by Nelson, D. M., Jaber-Hijazi, F., et al.
Fig.6.A

-
WB
-
Collected and cropped from Genome Biol by CiteAb, provided under a CC-BY license
Image 1 of 15
In Nat Commun on 8 September 2015 by Morgner, J., Ghatak, S., et al.
Fig.5.F

-
WB
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 15
In Oncotarget on 20 April 2015 by Bhagirath, D., Zhao, X., et al.
Fig.1.A

-
WB
-
Homo sapiens (Human)
Collected and cropped from Oncotarget by CiteAb, provided under a CC-BY license
Image 1 of 15
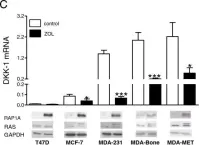
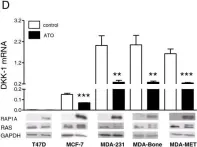
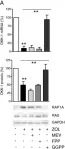
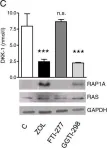
In Breast Cancer Res on 14 February 2014 by Rachner, T. D., Göbel, A., et al.
Fig.1.C

-
WB
-
Collected and cropped from Breast Cancer Res by CiteAb, provided under a CC-BY license
Image 1 of 15
In Breast Cancer Res on 14 February 2014 by Rachner, T. D., Göbel, A., et al.
Fig.1.D

-
WB
-
Collected and cropped from Breast Cancer Res by CiteAb, provided under a CC-BY license
Image 1 of 15
In Breast Cancer Res on 14 February 2014 by Rachner, T. D., Göbel, A., et al.
Fig.3.A

-
WB
-
Collected and cropped from Breast Cancer Res by CiteAb, provided under a CC-BY license
Image 1 of 15
In Breast Cancer Res on 14 February 2014 by Rachner, T. D., Göbel, A., et al.
Fig.3.C

-
WB
-
Collected and cropped from Breast Cancer Res by CiteAb, provided under a CC-BY license
Image 1 of 15
In PLoS One on 23 March 2012 by Yu, Z., Sato, S., et al.
Fig.3.D

-
WB
-
Homo sapiens (Human)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 15
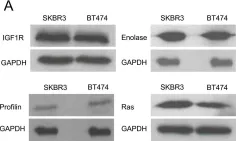
In BMC Cancer on 15 June 2010 by Kulkarni, Y. M., Suarez, V., et al.
Fig.6.A

-
WB
-
Homo sapiens (Human)
Collected and cropped from BMC Cancer by CiteAb, provided under a CC-BY license
Image 1 of 15