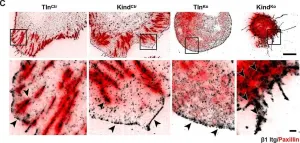
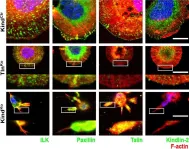
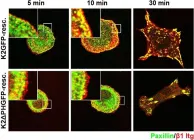
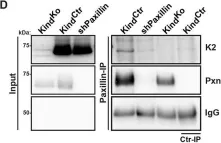
During animal development, cell-ECM adhesion mediated by integrins is required for the assembly and maintenance of tissues and organs. It can either be transient or stable and requires linking integrins to the cytoskeleton. Talin can link integrins to actin either directly through its actin-binding sites (ABSs) or indirectly by recruiting downstream actin-binding molecules. In Drosophila, talin's ABS3 domain is essential for biological functions, but its role remains unknown in mammalian systems. Here, we investigate the role of direct talin-mediated actin linkage in mammals by generating a mouse model containing point mutations in talin's ABS3 domain. We find that mutant mice exhibit early developmental defects and die midway through embryogenesis. Primary mouse embryonic fibroblasts generated from mutants form prominent focal adhesions but show defective consolidation and maturation. Adhesion dynamics, cell spreading, actin dynamics and organization, and traction force generation are also impacted in mutants, which affect processes such as cell migration that impinge on multiple events during early mouse embryogenesis. Overall, our work provides key mechanistic insights into how direct coupling of ECM to actin through talin has specific and critical roles in controlling adhesion dynamics required for early mammalian development.
© 2025. The Author(s).
Product Citations: 234
In Communications Biology on 21 June 2025 by Deng, W., Carr, R., et al.
-
Cell Biology
-
Stem Cells and Developmental Biology
In JCI Insight on 24 March 2025 by Waich, S., Kreidl, K., et al.
The osteo-oto-hepato-enteric (O2HE) syndrome is a severe autosomal recessive disease ascribed to loss-of-function mutations in the Unc-45 myosin chaperone A (UNC45A) gene. The clinical spectrum includes bone fragility, hearing loss, cholestasis, and life-threatening diarrhea associated with microvillus inclusion disease-like enteropathy. Here, we present molecular and functional analysis of the UNC45A c.710T>C (p.Leu237Pro) missense variant, which revealed a unique pathogenicity compared with other genetic variants causing UNC45A deficiency. The UNC45A p.Leu237Pro mutant retained chaperone activity, prevented myosin aggregation, and supported proper nonmuscle myosin II (NMII) filament formation in patient fibroblasts and human osteosarcoma (U2OS) cells. However, the mutant formed atypically stable oligomers and prevented chaperone-myosin complex dissociation, thereby inhibiting NMII functions. Similar to biallelic UNC45A deficiency, this resulted in impaired intracellular trafficking, defective recycling, and abnormal retention of transferrin at various endocytic sites. In particular, coexpression of wild-type protein attenuated the pathogenic effects of the variant by inhibiting excessive oligomer formation. Our results elucidate the pathogenic mechanisms and recessive characteristics of this variant and may aid in the development of targeted therapies.
-
WB
-
Homo sapiens (Human)
Preprint on BioRxiv : the Preprint Server for Biology on 23 December 2024 by Pedraza, N., Rocandio, D., et al.
During nervous system development, the interplay between cell cycle regulation and neurogenesis is fundamental to achieve the correct timing for neuronal differentiation. However, the molecular players regulating this transition are poorly understood. Among these, the cell-cycle regulatory cyclins and their cyclin-dependent kinases (Cdks) play a pivotal role. In the present work we uncover an unknown function of cyclin D1 (Ccnd1) during cortex development which is independent of cell cycle regulation and that relies on its cytoplasmic localization and membrane association. We show that Ccnd1 is localized in the cytoplasm of the radial glial process (RGP) of neuron progenitors in different regions of the developing brain, including the cortex. Cytoplasmic Ccnd1 is enriched at the distal tip of the RGP, adjacent to the meningeal basement membrane, and overlaps with β1-integrin at the plasma membrane. CCND1 knock-out animals show an abnormal cortical layering in which the distribution of Tbr2+ and Ctip2+ cells are affected without displaying proliferation defects. This is consistent with a cytoplasmic function of Ccnd1 as overexpression by in utero electroporation of a dominant negative Ccnd1, unable to activate Cdks, and targeted to the cytoplasmic membranes, reproduces some of these Tbr2 and Ctip2 defects. Finally, we provide evidence that cytoplasmic Ccnd1 affects neuron morphology and that it is required for the proper detachment of the RGP from the meningeal basement membrane by a mechanism involving the phosphorylation of the integrin effector protein paxillin. Hence, we propose that Ccnd1 has an important cytoplasmic function for cortical development independently of cell cycle regulation. Significant Statement A key developmental step during nervous system formation is the transition from proliferating progenitors to postmitotic neurons. However, the molecular mechanisms regulating this process are not fully understood. Cyclin D1 (Ccnd1) is a canonical regulator of cell cycle in the cell nucleus. Surprisingly, we show that Ccnd1 is also located in the radial glial process of neuron progenitors and associated to the plasma membrane in different regions of the developing mouse brain. We uncover a novel function for this cytoplasmic Ccnd1 and show that it is required for proper cortical layering, independent of cell cycle regulation. Mechanistically, we provide evidence that this function is mediated by the integrin effector paxillin. We propose therefore that cytoplasmic Ccnd1 is important for cortex development independent of cell cycle regulation.
-
WB
-
Cell Biology
-
Neuroscience
Decisive role of mDia-family formins in cell cortex function of highly adherent cells.
In Science Advances on 1 November 2024 by Scholz, J., Stephan, T., et al.
Cortical formins, pivotal for the assembly of linear actin filaments beneath the membrane, exert only minor effects on unconfined cell migration of weakly and moderately adherent cells. However, their impact on migration and mechanostability of highly adherent cells remains poorly understood. Here, we demonstrate that loss of cortical actin filaments generated by the formins mDia1 and mDia3 drastically compromises cell migration and mechanics in highly adherent fibroblasts. Biophysical analysis of the mechanical properties of the mutant cells revealed a markedly softened cell cortex in the poorly adherent state. Unexpectedly, in the highly adherent state, associated with a hyperstretched morphology with exaggerated focal adhesions and prominent high-strain stress fibers, they exhibited even higher cortical tension compared to control. Notably, misguidance of intracellular forces, frequently accompanied by stress-fiber rupture, culminated in the formation of tension- and contractility-induced macroapertures, which was instantly followed by excessive lamellipodial protrusion at the periphery, providing critical insights into mechanotransduction of mechanically stressed and highly adherent cells.
Kinesin-1 mediates proper ER folding of the CaV1.2 channel and maintains mouse glucose homeostasis.
In EMBO Reports on 1 November 2024 by Tanaka, Y., Farkhondeh, A., et al.
Glucose-stimulated insulin secretion (GSIS) from pancreatic beta cells is a principal mechanism for systemic glucose homeostasis, of which regulatory mechanisms are still unclear. Here we show that kinesin molecular motor KIF5B is essential for GSIS through maintaining the voltage-gated calcium channel CaV1.2 levels, by facilitating an Hsp70-to-Hsp90 chaperone exchange to pass through the quality control in the endoplasmic reticulum (ER). Phenotypic analyses of KIF5B conditional knockout (cKO) mouse beta cells revealed significant abolishment of glucose-stimulated calcium transients, which altered the behaviors of insulin granules via abnormally stabilized cortical F-actin. KIF5B and Hsp90 colocalize to microdroplets on ER sheets, where CaV1.2 but not Kir6.2 is accumulated. In the absence of KIF5B, CaV1.2 fails to be transferred from Hsp70 to Hsp90 via STIP1, and is likely degraded via the proteasomal pathway. KIF5B and Hsc70 overexpression increased CaV1.2 expression via enhancing its chaperone binding. Thus, ER sheets may serve as the place of KIF5B- and Hsp90-dependent chaperone exchange, which predominantly facilitates CaV1.2 production in beta cells and properly enterprises GSIS against diabetes.
© 2024. The Author(s).
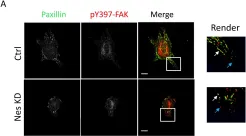
In Cells on 29 September 2022 by Wang, R., Khan, S., et al.
Fig.2.A

-
ICC-IF
-
Homo sapiens (Human)
Collected and cropped from Cells by CiteAb, provided under a CC-BY license
Image 1 of 21
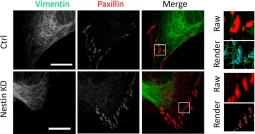
In Cells on 29 September 2022 by Wang, R., Khan, S., et al.
Fig.3.C

-
ICC-IF
-
Homo sapiens (Human)
Collected and cropped from Cells by CiteAb, provided under a CC-BY license
Image 1 of 21
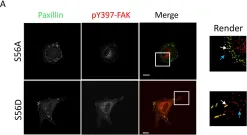
In Cells on 29 September 2022 by Wang, R., Khan, S., et al.
Fig.5.A

-
ICC-IF
-
Homo sapiens (Human)
Collected and cropped from Cells by CiteAb, provided under a CC-BY license
Image 1 of 21
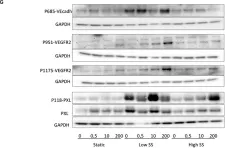
In Front Physiol on 20 March 2021 by Vion, A. C., Perovic, T., et al.
Fig.3.G

-
WB
-
Homo sapiens (Human)
Collected and cropped from Front Physiol by CiteAb, provided under a CC-BY license
Image 1 of 21
In Elife on 28 September 2020 by Bressan, C., Pecora, A., et al.
Fig.6.C

-
WB
-
Mus musculus (House mouse)
Collected and cropped from Elife by CiteAb, provided under a CC-BY license
Image 1 of 21
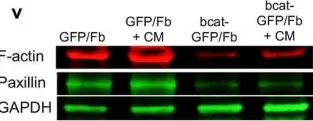
In Signal Transduct Target Ther on 31 December 2019 by Liu, T., Zhou, L., et al.
Fig.1.V

-
WB
-
Collected and cropped from Signal Transduct Target Ther by CiteAb, provided under a CC-BY license
Image 1 of 21
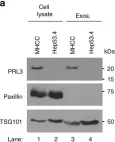
In Nat Commun on 6 June 2019 by Thura, M., Al-Aidaroos, A. Q., et al.
Fig.3.A

-
WB
-
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 21
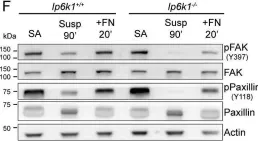
In Elife on 16 January 2017 by Benito-Jardón, M., Klapproth, S., et al.
Fig.1.C

-
IF
-
Mus musculus (House mouse)
Collected and cropped from Elife by CiteAb, provided under a CC-BY license
Image 1 of 21
In Cell Signal on 1 August 2016 by Jadav, R. S., Kumar, D., et al.
Fig.2.F

-
WB
-
Collected and cropped from Cell Signal by CiteAb, provided under a CC-BY license
Image 1 of 21
In PLoS One on 10 March 2016 by Hirth, S., Bühler, A., et al.
Fig.3.H

-
WB
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 21
In Elife on 27 January 2016 by Theodosiou, M., Widmaier, M., et al.
Fig.5.C

-
WB
-
Mus musculus (House mouse)
Collected and cropped from Elife by CiteAb, provided under a CC-BY license
Image 1 of 21
In Elife on 27 January 2016 by Theodosiou, M., Widmaier, M., et al.
Fig.1.B

-
ICC-IF
-
Mus musculus (House mouse)
Collected and cropped from Elife by CiteAb, provided under a CC-BY license
Image 1 of 21
In Elife on 27 January 2016 by Theodosiou, M., Widmaier, M., et al.
Fig.5.B

-
WB
-
Mus musculus (House mouse)
Collected and cropped from Elife by CiteAb, provided under a CC-BY license
Image 1 of 21
In Elife on 27 January 2016 by Theodosiou, M., Widmaier, M., et al.
Fig.3.C

-
IHC-IF
-
Mus musculus (House mouse)
Collected and cropped from Elife by CiteAb, provided under a CC-BY license
Image 1 of 21
In Elife on 27 January 2016 by Theodosiou, M., Widmaier, M., et al.
Fig.3.A

-
ICC-IF
-
Mus musculus (House mouse)
Collected and cropped from Elife by CiteAb, provided under a CC-BY license
Image 1 of 21
In Elife on 27 January 2016 by Theodosiou, M., Widmaier, M., et al.
Fig.4.F

-
IHC-IF
-
Mus musculus (House mouse)
Collected and cropped from Elife by CiteAb, provided under a CC-BY license
Image 1 of 21
In Elife on 27 January 2016 by Theodosiou, M., Widmaier, M., et al.
Fig.4.D

-
WB
-
Mus musculus (House mouse)
Collected and cropped from Elife by CiteAb, provided under a CC-BY license
Image 1 of 21
In Cancer Cell Int on 9 June 2015 by Moazzam, M., Ye, L., et al.
Fig.5.B

-
WB
-
Homo sapiens (Human)
Collected and cropped from Cancer Cell Int by CiteAb, provided under a CC-BY license
Image 1 of 21
In PLoS One on 20 December 2014 by Ng, D. H., Humphries, J. D., et al.
Fig.3.A

-
WB
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 21
In Nat Commun on 7 August 2013 by Vyas, S., Chesarone-Cataldo, M., et al.
Fig.6.C

-
ICC-IF
-
Homo sapiens (Human)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 21
In Breast Cancer Res on 28 February 2012 by Lahlou, H., Sanguin-Gendreau, V., et al.
Fig.4.A

-
WB
-
Collected and cropped from Breast Cancer Res by CiteAb, provided under a CC-BY license
Image 1 of 21