Caveolae are organizing centers for cellular signal transduction in endothelial cells (ED) and smooth muscle cells (SMCs) in the blood vessels. Myography was used to investigate the effects of a caveolar disruption using methyl-β-cyclodextrin (MBCD) on maxi-K channels in rat carotid arteries. Incubation of carotid segments with MBCD augmented contractions in response to BaK (chemical channel agonist) but not those induced by depolarizing high potassium physiological saline (KPSS). In contrast, incubation with cholesterol-saturated MBCD (Ch-MBCD) abolished the effects of MBCD. Mechanical removal of endothelial cells by MBCD triggered a small contraction in response to BaK. Incubation with nitroarginine methyl ester (L-NAME) inhibited nitric oxide (NO) release, causing increased contractions in response to BaK, and this effect was reversed by pretreatment with MBCD. These results suggest that MBCD inhibits endothelial NO release. Contrastingly, inhibition of maxi-K channels with iberiotoxin enhanced contractions in response to BaK. Likewise, L-NAME decreased the contractile effect of iberiotoxin, as in the ED-denuded arteries. Transmission electron microscopy (TEM) showed the presence and absence of caveolae in intact blood vessels before and after MBCD treatment, respectively, whereas histology confirmed ED removal after the treatment. Caveolar disruption using MBCD impairs ED-dependent relaxation by inhibiting the release of NO from the ED and altered the contractility of SMCs independent of the ED due to reduced contribution of maxi-K channels to the SMC membrane potential, causing depolarization and increasing carotid artery contraction. These findings might help to understand the physiological role of the maxi-K channels in rat carotid arteries.
© 2022. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
Product Citations: 13
In Environmental Science and Pollution Research International on 1 September 2022 by Albrakati, A.
-
WB
-
Rattus norvegicus (Rat)
-
Cardiovascular biology
In The FASEB Journal on 1 December 2020 by Torices, S., Roberts, S. A., et al.
HIV-1 enters the brain by altering properties of the blood-brain barrier (BBB). Recent evidence indicates that among cells of the BBB, pericytes are prone to HIV-1 infection. Occludin (ocln) and caveolin-1 (cav-1) are critical determinants of BBB integrity that can regulate barrier properties of the BBB in response to HIV-1 infection. Additionally, Alix is an early acting endosomal factor involved in HIV-1 budding from the cells. The aim of the present study was to evaluate the role of cav-1, ocln, and Alix in HIV-1 infection of brain pericytes. Our results indicated that cav-1, ocln, and Alix form a multi-protein complex in which they cross-regulate each other's expression. Importantly, the stability of this complex was affected by HIV-1 infection. Modifications of the complex resulted in diminished HIV-1 infection and alterations of the cytokine profile produced by brain pericytes. These results identify a novel mechanism involved in HIV-1 infection contributing to a better understanding of the HIV-1 pathology and the associated neuroinflammatory responses.
© 2020 The Authors. The FASEB Journal published by Wiley Periodicals LLC on behalf of Federation of American Societies for Experimental Biology.
-
WB
-
Homo sapiens (Human)
-
Immunology and Microbiology
In Journal of Cachexia, Sarcopenia and Muscle on 1 June 2020 by Shah, D. S., Nisr, R. B., et al.
Caveolin-3 (Cav3) is the principal structural component of caveolae in skeletal muscle. Dominant pathogenic mutations in the Cav3 gene, such as the Limb Girdle Muscular Dystrophy-1C (LGMD1C) P104L mutation, result in substantial loss of Cav3 and myopathic changes characterized by muscle weakness and wasting. We hypothesize such myopathy may also be associated with disturbances in mitochondrial biology. Herein, we report studies assessing the effects of Cav3 deficiency on mitochondrial form and function in skeletal muscle cells.
L6 myoblasts were stably transfected with Cav3P104L or expression of native Cav3 repressed by shRNA or CRISPR/Cas9 genome editing prior to performing fixed/live cell imaging of mitochondrial morphology, subcellular fractionation and immunoblotting, or analysis of real time mitochondrial respiration. Skeletal muscle from wild-type and Cav3-/- mice was processed for analysis of mitochondrial proteins by immunoblotting.
Caveolin-3 was detected in mitochondrial-enriched membranes isolated from mouse gastrocnemius muscle and L6 myoblasts. Expression of Cav3P104L in L6 myoblasts led to its targeting to the Golgi and loss of native Cav3 (>95%), including that associated with mitochondrial membranes. Cav3P104L reduced mitochondrial mass and induced fragmentation of the mitochondrial network that was associated with significant loss of proteins involved in mitochondrial biogenesis, respiration, morphology, and redox function [i.e. PGC1α, succinate dehyrdogenase (SDHA), ANT1, MFN2, OPA1, and MnSOD). Furthermore, Cav3P104L myoblasts exhibited increased mitochondrial cholesterol and loss of cardiolipin. Consistent with these changes, Cav3P104L expression reduced mitochondrial respiratory capacity and increased myocellular superoxide production. These morphological, biochemical, and functional mitochondrial changes were phenocopied in myoblasts in which Cav3 had been silenced/knocked-out using shRNA or CRISPR. Reduced mitochondrial mass, PGC1α, SDHA, ANT1, and MnSOD were also demonstrable in Cav3-/- mouse gastrocnemius. Strikingly, Cav3 re-expression in Cav3KO myoblasts restored its mitochondrial association and facilitated reformation of a tubular mitochondrial network. Significantly, re-expression also mitigated changes in mitochondrial superoxide, cholesterol, and cardiolipin content and recovered cellular respiratory capacity.
Our results identify Cav3 as an important regulator of mitochondrial homeostasis and reveal that Cav3 deficiency in muscle cells associated with the Cav3P104L mutation invokes major disturbances in mitochondrial respiration and energy status that may contribute to the pathology of LGMD1C.
© 2020 The Authors. Journal of Cachexia, Sarcopenia and Muscle published by John Wiley & Sons Ltd on behalf of Society on Sarcopenia, Cachexia and Wasting Disorders.
-
WB
-
Cell Biology
In Arteriosclerosis, Thrombosis, and Vascular Biology on 1 June 2020 by Zhang, X., Ramírez, C. M., et al.
Endothelial Cav-1 (caveolin-1) expression plays a relevant role during atherogenesis by controlling NO production, vascular inflammation, LDL (low-density lipoprotein) transcytosis, and extracellular matrix remodeling. Additional studies have identified cholesterol-rich membrane domains as important regulators of autophagy by recruiting ATGs (autophagy-related proteins) to the plasma membrane. Here, we investigate how the expression of Cav-1 in the aortic endothelium influences autophagy and whether enhanced autophagy contributes to the atheroprotective phenotype observed in Cav-1-deficient mice. Approach and Results: To analyze the impact of Cav-1 deficiency on regulation of autophagy in the aortic endothelium during the progression of atherosclerosis, we fed Ldlr-/- and Cav-1-/-Ldlr-/- mice a Western diet and assessed autophagy in the vasculature. We observe that the absence of Cav-1 promotes autophagy activation in athero-prone areas of the aortic endothelium by enhancing autophagic flux. Mechanistically, we found that Cav-1 interacts with the ATG5-ATG12 complex and influences the cellular localization of autophagosome components in lipid rafts, which controls the autophagosome formation and autophagic flux. Pharmacological inhibition of autophagy attenuates the atheroprotection observed in Cav-1-/- mice by increasing endothelial inflammation and macrophage recruitment, identifying a novel molecular mechanism by which Cav-1 deficiency protects against the progression of atherosclerosis.
These results identify Cav-1 as a relevant regulator of autophagy in the aortic endothelium and demonstrate that pharmacological suppression of autophagic flux in Cav-1-deficient mice attenuates the atheroprotection observed in Cav-1-/- mice. Additionally, these findings suggest that activation of endothelial autophagy by blocking Cav-1 might provide a potential therapeutic strategy for cardiovascular diseases including atherosclerosis.
-
WB
-
Cardiovascular biology
-
Cell Biology
-
Immunology and Microbiology
In Scientific Reports on 21 August 2019 by Nanni, P., Landuzzi, L., et al.
Standard therapy of osteosarcoma (OS) and Ewing sarcoma (EW) rests on cytotoxic regimes, which are largely unsuccessful in advanced patients. Preclinical models are needed to break this impasse. A panel of patient-derived xenografts (PDX) was established by implantation of fresh, surgically resected osteosarcoma (OS) and Ewing sarcoma (EW) in NSG mice. Engraftment was obtained in 22 of 61 OS (36%) and 7 of 29 EW (24%). The success rate in establishing primary cell cultures from OS was lower than the percentage of PDX engraftment in mice, whereas the reverse was observed for EW; the implementation of both in vivo and in vitro seeding increased the proportion of patients yielding at least one workable model. The establishment of in vitro cultures from PDX was highly efficient in both tumor types, reaching 100% for EW. Morphological and immunohistochemical (SATB2, P-glycoprotein 1, CD99, caveolin 1) studies and gene expression profiling showed a remarkable similarity between patient's tumor and PDX, which was maintained over several passages in mice, whereas cell cultures displayed a lower correlation with human samples. Genes differentially expressed between OS original tumor and PDX mostly belonged to leuykocyte-specific pathways, as human infiltrate is gradually replaced by murine leukocytes during growth in mice. In EW, which contained scant infiltrates, no gene was differentially expressed between the original tumor and the PDX. A novel therapeutic combination of anti-CD99 diabody C7 and irinotecan was tested against two EW PDX; both drugs inhibited PDX growth, the addition of anti-CD99 was beneficial when chemotherapy alone was less effective. The panel of OS and EW PDX faithfully mirrored morphologic and genetic features of bone sarcomas, representing reliable models to test therapeutic approaches.
-
IHC
In J Cachexia Sarcopenia Muscle on 1 June 2020 by Shah, D. S., Nisr, R. B., et al.
Fig.4.A
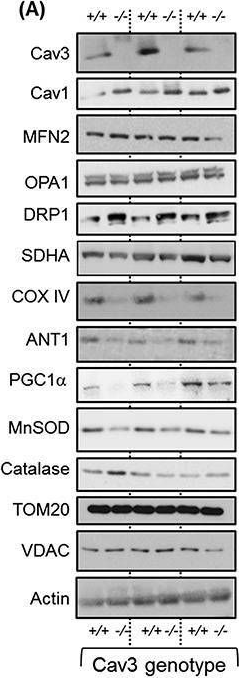
-
WB
-
Collected and cropped from J Cachexia Sarcopenia Muscle by CiteAb, provided under a CC-BY license
Image 1 of 1
