Altered cellular metabolism has been associated with the acquisition of invasive phenotypes during metastasis. To study this, we combined a genetically engineered mouse model of mammary carcinoma with syngeneic transplantation and primary tumor resection to generate isogenic cells from primary tumors and their corresponding lung micrometastases. Metabolic analyses indicated that micrometastatic cells increase proline production at the expense of glutathione synthesis, leading to a reduction in total glutathione levels. Micrometastatic cells also have altered sphingomyelin metabolism, leading to increased intracellular levels of specific ceramides. The combination of these metabolic adaptations alters extracellular vesicle (EV) production to render the microenvironment more permissive for invasion. Indeed, micrometastatic cells shut down Rab27-dependent production of EVs and, instead, switch on neutral sphingomyelinase-2 (nSM2)-dependent EV release. EVs released in an nSM2-dependent manner from micrometastatic cells, in turn, influence the ability of fibroblasts to deposit extracellular matrix, which promotes cancer cell invasiveness. These data provide evidence that metabolic rewiring drives invasive processes in metastasis by influencing EV release.
© 2025 Gounis et al.
Product Citations: 75
Metabolic adaptations of micrometastases alter EV production to generate invasive microenvironments.
In The Journal of Cell Biology on 4 August 2025 by Gounis, M., Campos, A., et al.
-
Biochemistry and Molecular biology
-
Cell Biology
In Experimental & Molecular Medicine on 1 April 2025 by Lee, H. J., Lim, S. H., et al.
Phospholipase D6 (PLD6) is a critical enzyme involved in mitochondrial fusion with a key role in spermatogenesis. However, the role of PLD6 in cancer remains unknown. Notably, Wnt signaling, energy metabolism and mitochondrial function show complex interactions in colorectal cancer (CRC) progression. Here we found that PLD6 is highly expressed in CRC and positively correlated with poor prognosis. We present a novel function of PLD6 in activating Wnt/β-catenin signaling by enhancing mitochondrial metabolism. PLD6 depletion suppresses the oncogenic properties of CRC cells and impairs mitochondrial respiration, leading to reduced mitochondrial length, membrane potential, calcium levels and reactive oxygen species. PLD6 depletion also disrupts mitochondrial metabolic reprogramming by inhibiting the tricarboxylic acid cycle and mitochondrial oxidative phosphorylation, resulting in altered intracellular levels of citrate and acetyl-CoA-both key modulators of Wnt/β-catenin activation. PLD6-mediated acetyl-CoA production enhances β-catenin stability by promoting its acetylation via the acetyltransferases CREB-binding protein and P300/CREB-binding-protein-associated factor. Consequently, PLD6 ablation reduces cancer stem cell-associated gene expression downstream of Wnt/β-catenin signaling, suppressing stem-like traits and chemoresistance to 5-fluorouracil. Furthermore, PLD6 depletion attenuates CRC tumorigenesis in both subcutaneous and orthotopic tumor models. Overall, PLD6 acts as an oncogenic switch by promoting mitochondria-mediated retrograde signaling, thereby regulating Wnt signaling in CRC.
© 2025. The Author(s).
-
Biochemistry and Molecular biology
-
Cancer Research
-
Cell Biology
In Experimental & Molecular Medicine on 1 April 2025 by Hong, K. S., Ryu, K. J., et al.
Mitogen- and stress-activated protein kinase 1 (MSK1), a Ser/Thr kinase, phosphorylates nuclear proteins to increase their stability and DNA-binding affinity. Despite the role of MSK1 in promoting cancer progression in colorectal cancer (CRC), the precise molecular mechanisms remain unelucidated. Here we show that MSK1 expression induces the epithelial-mesenchymal transition (EMT) process and increases CRC cell metastasis. Furthermore, we discovered that MSK1 interacts with Snail, a key EMT regulator, and increases its stability by inhibiting ubiquitin-mediated proteasomal degradation. Importantly, MSK1 increased Snail protein stability by promoting deubiquitination rather than inhibiting its ubiquitination. Finally, we identified USP5 as an essential deubiquitinase that binds to Snail protein phosphorylated by MSK1. Based on the experimental data, in CRC, MSK1-Snail-USP5 axis can promote EMT and metastasis of CRC. Together, our findings provide potential biomarkers and novel therapeutic targets for further research in CRC.
© 2025. The Author(s).
-
Biochemistry and Molecular biology
-
Cancer Research
In Matrix Biology : Journal of the International Society for Matrix Biology on 1 February 2025 by Hossain, A. S., Clarin, M. T. R. D. C., et al.
Fibrillin-1, an extracellular matrix (ECM) protein encoded by the FBN1 gene, serves as a microfibril scaffold crucial for elastic fiber formation and homeostasis in pliable tissue such as the skin. Aside from causing Marfan syndrome, some mutations in FBN1 result in scleroderma, marked by hardened and thicker skin which limits joint mobility. Here, we describe a tight skin phenotype in the Fbn1G234D/G234D mice carrying a corresponding variant of FBN1 in the hybrid1 domain that was identified in a patient with familial aortic dissection. Unlike scleroderma, skin thickness and collagen fiber abundance do not change in the Fbn1G234D/G234D mutant skin. Instead, increased collagen cross-links were observed. In addition, short elastic fibers were sparsely located underneath the panniculus muscle layer, and an abundance of thin, aberrant elastic fibers was increased within the subcutaneous fascia, which may have tightened skin attachment to the underlying skeletal muscle. Structurally, Fbn1G234D/G234D microfibrils have a disrupted shoulder region that shares similarities with hybrid1 deletion mutant microfibrils. We then demonstrate the consequence of fibrillin-1 G234D mutation on dermal fibroblast functions. Mutant primary fibroblasts produce fewer elastic fibers, exhibit slower migration and increased cell stiffness. Moreover, secretome from mutant fibroblasts are marked by enhanced secretion of ECM, ECM-modifying enzymes, proteoglycans and cytokines, which are pro-tissue repair/fibrogenic. The transcriptome of mutant fibroblasts displays an increased expression of myogenic developmental and immune-related genes. Our study proposes that imbalanced ECM homeostasis due to a fibrillin-1 G234D mutation impacts fibroblast properties with potential ramifications on skin function.
Copyright © 2024. Published by Elsevier B.V.
Modulators of Alpha-2 Macroglobulin Upregulation by High Glucose in Glomerular Mesangial Cells.
In Biomolecules on 13 November 2024 by Trink, J., Li, R., et al.
Up to 40% of patients with diabetes mellitus will develop diabetic kidney disease (DKD), characterized pathologically by the accumulation of extracellular matrix proteins, which leads to the loss of kidney function over time. Our previous studies showed that the pan-protease inhibitor alpha 2-macroglobulin (A2M) is increased in DKD and is a critical regulator of the fibrotic response in glomerular mesangial cells (MC), an initial site of injury during DKD development. How A2M is regulated by high glucose (HG) has not yet been elucidated and is the focus of this investigation. Using serial deletions of the full A2M promoter, we identified the -405 bp region as HG-responsive in MC. Site-directed mutagenesis, siRNA, and ChIP studies showed that the transcription factor, nuclear factor of activated T cells 5 (NFAT5), regulated A2M promoter activity and protein expression in response to HG. Forkhead box P1 (FOXP1) served as a cooperative binding partner for NFAT5, required for A2M upregulation. Lastly, we showed that Smad3, known for its role in kidney fibrosis, regulated A2M promoter activity and protein production independently of HG. The importance of NFAT5, FOXP1, and Smad3 in A2M regulation was confirmed in ex vivo studies using isolated glomeruli. In conclusion, Smad3 is required for basal and HG-induced A2M expression, while NFAT5 and FOXP1 cooperatively regulate increased A2M transcription in response to HG. Inhibition of NFAT5/FOXP1 will be further evaluated as a potential therapeutic strategy to inhibit A2M production and attenuate profibrotic signaling in DKD.
-
WB
-
Biochemistry and Molecular biology
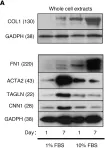
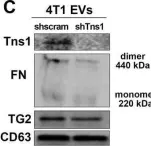
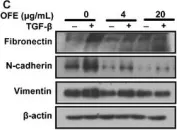
In Mol Cancer on 11 October 2024 by Ryu, K. J., Lee, K. W., et al.
Fig.4.E

-
WB
-
Collected and cropped from Mol Cancer by CiteAb, provided under a CC-BY license
Image 1 of 11
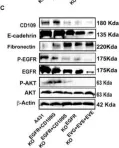
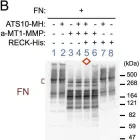
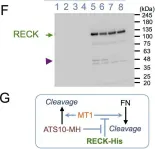
In JCI Insight on 23 April 2024 by Papaioannou, I., Dritsoula, A., et al.
Fig.2.A

-
WB
-
Collected and cropped from JCI Insight by CiteAb, provided under a CC-BY license
Image 1 of 11
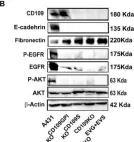
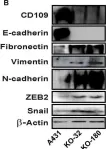
In Cancers (Basel) on 28 July 2022 by Zhou, S., Hassan, A., et al.
Fig.6.C

-
WB
-
Collected and cropped from Cancers (Basel) by CiteAb, provided under a CC-BY license
Image 1 of 11
In Cancers (Basel) on 28 July 2022 by Zhou, S., Hassan, A., et al.
Fig.6.B

-
WB
-
Collected and cropped from Cancers (Basel) by CiteAb, provided under a CC-BY license
Image 1 of 11
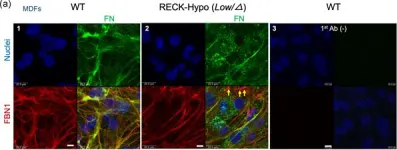
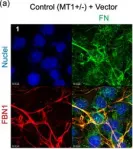
In J Cell Physiol on 1 March 2021 by Matsuzaki, T., Keene, D. R., et al.
Fig.2.A

-
ICC-IF
-
Collected and cropped from J Cell Physiol by CiteAb, provided under a CC-BY license
Image 1 of 11
In J Cell Physiol on 1 March 2021 by Matsuzaki, T., Keene, D. R., et al.
Fig.6.A

-
ICC-IF
-
Collected and cropped from J Cell Physiol by CiteAb, provided under a CC-BY license
Image 1 of 11
In Oncogenesis on 13 February 2020 by Shinde, A., Paez, J. S., et al.
Fig.6.C

-
WB
-
Collected and cropped from Oncogenesis by CiteAb, provided under a CC-BY license
Image 1 of 11
In Sci Rep on 6 November 2019 by Zhou, S., da Silva, S. D., et al.
Fig.6.B

-
WB
-
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 11
In Biol Open on 4 October 2018 by Matsuzaki, T., Kitayama, H., et al.
Fig.4.B

-
WB
-
Collected and cropped from Biol Open by CiteAb, provided under a CC-BY license
Image 1 of 11
In Biol Open on 4 October 2018 by Matsuzaki, T., Kitayama, H., et al.
Fig.4.F

-
WB
-
Collected and cropped from Biol Open by CiteAb, provided under a CC-BY license
Image 1 of 11
In Chin Med on 2 July 2015 by Chang, L., Jia, S., et al.
Fig.3.C

-
WB
-
Collected and cropped from Chin Med by CiteAb, provided under a CC-BY license
Image 1 of 11