Focal adhesions (FAs) are force-bearing multiprotein complexes, whose nanoscale organization and signaling are essential for cell growth and differentiation. However, the specific organization of FA components to exert spatiotemporal activation of FA proteins for force sensing and transduction remains unclear. In this study, we unveil the intricacies of FA protein nanoarchitecture and that its dynamics are coordinated by a molecular scaffold protein, BNIP-2, to initiate downstream signal transduction for cardiomyoblast differentiation. Within the FAs, BNIP-2 regulates the nano-organization of focal adhesion kinase (FAK), and the dynamics of FAK, paxillin, and vinculin. Depletion of BNIP-2 resulted in altered focal adhesion numbers and sizes per cell, reduced traction force, and decreased FA sensitivity for mechanosensing. At the molecular level, the loss of BNIP-2 disrupted the FAK-paxillin signaling axis, where FAK inhibition reproduces the effects of BNIP-2 loss by impairing the phosphorylation of both FAK and paxillin. Mechanistically, BNIP-2 preferentially binds to constitutively active FAK and acts as a molecular scaffold to mediate interactions between FAK and paxillin and between paxillin and vinculin. We have validated BNIP-2's role in the FAK-paxillin signaling axis in human embryonic stem cells (hESC). Furthermore, we showed that depletion of BNIP-2 resulted in changes in signature gene targets at the cardiac progenitor stage of differentiation. In summary, we showed that the intricate interplay of FA nanoarchitecture and dynamics, governed by BNIP-2, is crucial for force transduction and biochemical signaling in driving cardiomyoblast differentiation.
Product Citations: 99
In ACS Applied Materials Interfaces on 22 January 2025 by Xiao, J., Ang, J. W., et al.
Role of the androgen receptor in melanoma aggressiveness.
In Cell Death & Disease on 21 January 2025 by Di Donato, M., Cristiani, C. M., et al.
Malignant melanoma represents the fifth most common cancer in the world and its incidence is rising. Novel therapies targeting receptor tyrosine kinases, kinases and immune checkpoints have been employed with a significant improvement of the overall survival and long-term disease containment. Nevertheless, the disease often progresses and becomes resistant to the therapies. As such, the discovery of new targets and drugs for advanced melanoma still remains a difficult task. Gender disparities, with a female advantage in melanoma incidence and outcome, have been reported. Although emerging studies support the pro-tumorigenic role of androgen/androgen receptor axis in melanoma, the molecular bases of such evidence are still under intense investigation. We now report that ligand activation of the androgen receptor drives melanoma invasiveness and its escape from natural killer-mediated cytotoxic effect. By combining different experimental approaches, we observe that melanoma escape is mediated by the androgen-triggered shedding of the surface molecule MICA. Specific blockade of ADAM10 or androgen receptor impairs the androgen-induced MICA shedding and melanoma immune-escape. Further, the increase in MICA serum levels correlates with a poor outcome in melanoma patients treated with the anti-PD-1 monoclonal antibody, pembrolizumab. At last, melanoma cells depleted of the androgen receptor become more responsive to the most commonly used immunocheckpoint inhibitors, suggesting that the receptor dampens the immunotherapy efficacy. Taken together, our findings identify the androgen receptor as a diagnostic guidance in melanoma and support the repositioning of AR blockers in clinical management of patients.
© 2025. The Author(s).
-
Homo sapiens (Human)
-
Cancer Research
-
Cell Biology
-
Endocrinology and Physiology
A comparative analysis of paxillin and Hic-5 proximity interactomes.
In Cytoskeleton (Hoboken, N.J.) on 1 January 2025 by Brock, K., Alpha, K. M., et al.
Focal adhesions serve as structural and signaling hubs, facilitating bidirectional communication at the cell-extracellular matrix interface. Paxillin and the related Hic-5 (TGFβ1i1) are adaptor/scaffold proteins that recruit numerous structural and regulatory proteins to focal adhesions, where they perform both overlapping and discrete functions. In this study, paxillin and Hic-5 were expressed in U2OS osteosarcoma cells as biotin ligase (BioID2) fusion proteins and used as bait proteins for proximity-dependent biotinylation in order to directly compare their respective interactomes. The fusion proteins localized to both focal adhesions and the centrosome, resulting in biotinylation of components of each of these structures. Biotinylated proteins were purified and analyzed by mass spectrometry. The list of proximity interactors for paxillin and Hic-5 comprised numerous shared core focal adhesion proteins that likely contribute to their similar functions in cell adhesion and migration, as well as proteins unique to paxillin and Hic-5 that have been previously localized to focal adhesions, the centrosome, or the nucleus. Western blotting confirmed biotinylation and enrichment of FAK and vinculin, known interactors of Hic-5 and paxillin, as well as several potentially unique proximity interactors of Hic-5 and paxillin, including septin 7 and ponsin, respectively. Further investigation into the functional relationship between the unique interactors and Hic-5 or paxillin may yield novel insights into their distinct roles in cell migration.
© 2024 Wiley Periodicals LLC.
-
Homo sapiens (Human)
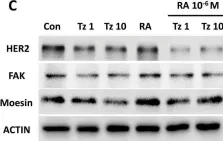
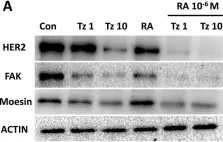
In International Journal of Molecular Sciences on 28 May 2024 by Mygind, K. J., Nikodemus, D., et al.
Desmoplasia is a common feature of aggressive cancers, driven by a complex interplay of protein production and degradation. Basigin is a type 1 integral membrane receptor secreted in exosomes or released by ectodomain shedding from the cell surface. Given that soluble basigin is increased in the circulation of patients with a poor cancer prognosis, we explored the putative role of the ADAM12-generated basigin ectodomain in cancer progression. We show that recombinant basigin ectodomain binds β1 integrin and stimulates gelatin degradation and the migration of cancer cells in a matrix metalloproteinase (MMP)- and β1-integrin-dependent manner. Subsequent in vitro and in vivo experiments demonstrated the altered expression of extracellular matrix proteins, including fibronectin and collagen type 5. Thus, we found increased deposits of collagen type 5 in the stroma of nude mice tumors of the human tumor cell line MCF7 expressing ADAM12-mimicking the desmoplastic response seen in human cancer. Our findings indicate a feedback loop between ADAM12 expression, basigin shedding, TGFβ signaling, and extracellular matrix (ECM) remodeling, which could be a mechanism by which ADAM12-generated basigin ectodomain contributes to the regulation of desmoplasia, a key feature in human cancer progression.
-
Cancer Research
Filamin A cooperates with the androgen receptor in preventing skeletal muscle senescence.
In Cell Death Discovery on 2 December 2023 by Di Donato, M., Moretti, A., et al.
Aging induces a slow and progressive decrease in muscle mass and function, causing sarcopenia. Androgens control muscle trophism and exert important anabolic functions through the binding to the androgen receptor. Therefore, analysis of the androgen receptor-mediated actions in skeletal muscle might provide new hints for a better understanding of sarcopenia pathogenesis. In this study, we report that expression of the androgen receptor in skeletal muscle biopsies from 20 subjects is higher in young, as compared with old subjects. Co-immunoprecipitation experiments reveal that the androgen receptor is complexed with filamin A mainly in young, that in old subjects. Therefore, we have in depth analyzed the role of such complex using C2C12 myoblasts that express a significant amount of the androgen receptor. In these cells, hormone stimulation rapidly triggers the assembly of the androgen receptor/filamin A complex. Such complex prevents the senescence induced by oxidative stress in C2C12 cells, as disruption of the androgen receptor/filamin A complex by Rh-2025u stapled peptide re-establishes the senescent phenotype in C2C12 cells. Simultaneously, androgen stimulation of C2C12 cells rapidly triggers the activation of various signaling effectors, including Rac1, focal adhesion kinase, and mitogen-activated kinases. Androgen receptor blockade by bicalutamide or perturbation of androgen receptor/filamin A complex by Rh-2025u stapled peptide both reverse the hormone activation of signaling effectors. These findings further reinforce the role of the androgen receptor and its extranuclear partners in the rapid hormone signaling that controls the functions of C2C12 cells. Further investigations are needed to promote clinical interventions that might ameliorate muscle cell function as well the clinical outcome of age-related frailty.
© 2023. The Author(s).
-
Endocrinology and Physiology
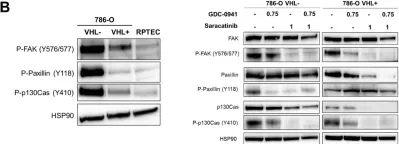
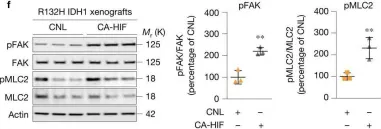
In JCI Insight on 8 December 2022 by Hayashi, S., Muraleedharan, C. K., et al.
Fig.4.E

-
WB
-
Collected and cropped from JCI Insight by CiteAb, provided under a CC-BY license
Image 1 of 23
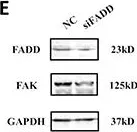
In Int J Mol Sci on 13 August 2022 by Saleh, B., Srikanth, K. D., et al.
Fig.2.A

-
WB
-
Collected and cropped from Int J Mol Sci by CiteAb, provided under a CC-BY license
Image 1 of 23
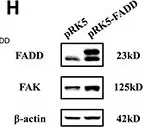
In Cell Death Dis on 14 July 2022 by Hou, S., Hao, X., et al.
Fig.5.C

-
WB
-
Collected and cropped from Cell Death Dis by CiteAb, provided under a CC-BY license
Image 1 of 23
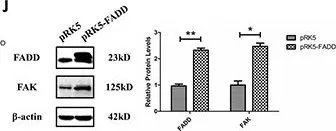
In Cell Death Dis on 14 July 2022 by Hou, S., Hao, X., et al.
Fig.5.D

-
WB
-
Collected and cropped from Cell Death Dis by CiteAb, provided under a CC-BY license
Image 1 of 23
In Cell Death Dis on 14 July 2022 by Hou, S., Hao, X., et al.
Fig.5.A

-
WB
-
Collected and cropped from Cell Death Dis by CiteAb, provided under a CC-BY license
Image 1 of 23
In Cell Death Dis on 14 July 2022 by Hou, S., Hao, X., et al.
Fig.5.B

-
WB
-
Collected and cropped from Cell Death Dis by CiteAb, provided under a CC-BY license
Image 1 of 23
In Sci Rep on 23 March 2022 by Frey, Y., Franz-Wachtel, M., et al.
Fig.1.D

-
WB
-
Homo sapiens (Human)
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 23
In Cells on 7 December 2021 by Gourley, S. L., Srikanth, K. D., et al.
Fig.3.B

-
WB
-
Collected and cropped from Cells by CiteAb, provided under a CC-BY license
Image 1 of 23
In Oncotarget on 10 July 2018 by Roelants, C., Giacosa, S., et al.
Fig.2.B

-
WB
-
Collected and cropped from Oncotarget by CiteAb, provided under a CC-BY license
Image 1 of 23
In Oncotarget on 29 May 2018 by Vanderhoeven, F., Redondo, A. L., et al.
Fig.10.C

-
WB
-
Collected and cropped from Oncotarget by CiteAb, provided under a CC-BY license
Image 1 of 23
In Oncotarget on 29 May 2018 by Vanderhoeven, F., Redondo, A. L., et al.
Fig.10.A

-
WB
-
Collected and cropped from Oncotarget by CiteAb, provided under a CC-BY license
Image 1 of 23
In J Pathol on 1 July 2017 by Alexopoulou, A. N., Lees, D. M., et al.
Fig.6.A

-
WB
-
Collected and cropped from J Pathol by CiteAb, provided under a CC-BY license
Image 1 of 23
In Nat Cell Biol on 1 December 2016 by Miroshnikova, Y. A., Mouw, J. K., et al.
Fig.4.F

-
WB
-
Collected and cropped from Nat Cell Biol by CiteAb, provided under a CC-BY license
Image 1 of 23
In Oncotarget on 9 August 2016 by Liu, Y., Cui, H., et al.
Fig.2.E

-
WB
-
Collected and cropped from Oncotarget by CiteAb, provided under a CC-BY license
Image 1 of 23
In Oncotarget on 9 August 2016 by Liu, Y., Cui, H., et al.
Fig.2.H

-
WB
-
Collected and cropped from Oncotarget by CiteAb, provided under a CC-BY license
Image 1 of 23
In Oncotarget on 9 August 2016 by Liu, Y., Cui, H., et al.
Fig.4.J

-
WB
-
Collected and cropped from Oncotarget by CiteAb, provided under a CC-BY license
Image 1 of 23
In Oncotarget on 9 August 2016 by Liu, Y., Cui, H., et al.
Fig.4.B

-
WB
-
Collected and cropped from Oncotarget by CiteAb, provided under a CC-BY license
Image 1 of 23
In Oncotarget on 9 August 2016 by Liu, Y., Cui, H., et al.
Fig.5.B

-
WB
-
Mus musculus (House mouse)
Collected and cropped from Oncotarget by CiteAb, provided under a CC-BY license
Image 1 of 23
In Aging (Albany NY) on 1 May 2016 by Segatto, I., Massarut, S., et al.
Fig.2.B

-
WB
-
Collected and cropped from Aging (Albany NY) by CiteAb, provided under a CC-BY license
Image 1 of 23
In J Cell Mol Med on 1 June 2014 by Flamini, M. I., Gauna, G. V., et al.
Fig.3.C

-
WB
-
Homo sapiens (Human)
Collected and cropped from J Cell Mol Med by CiteAb, provided under a CC-BY license
Image 1 of 23
In J Cell Mol Med on 1 June 2014 by Flamini, M. I., Gauna, G. V., et al.
Fig.4.C

-
WB
-
Homo sapiens (Human)
Collected and cropped from J Cell Mol Med by CiteAb, provided under a CC-BY license
Image 1 of 23
In J Cell Mol Med on 1 June 2014 by Flamini, M. I., Gauna, G. V., et al.
Fig.5.C

-
WB
-
Homo sapiens (Human)
Collected and cropped from J Cell Mol Med by CiteAb, provided under a CC-BY license
Image 1 of 23
In PLoS One on 27 August 2013 by Teräväinen, T. P., Myllymäki, S. M., et al.
Fig.6.E

-
WB
-
Canis lupus familiaris (Domestic dog)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 23