Mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene cause the recessive genetic disease cystic fibrosis, where the chloride transport across the apical membrane of epithelial cells mediated by the CFTR protein is impaired. CFTR protein trafficking to the plasma membrane (PM) is the result of a complex interplay between the secretory and membrane recycling pathways that control the number of channels present at the membrane. In addition, the ion transport activity of CFTR at the PM is modulated through post-translational protein modifications. Previously we described that spleen tyrosine kinase (SYK) phosphorylates a specific tyrosine residue in the nucleotide-binding domain 1 domain and this modification can regulate the PM abundance of CFTR. Here we identified the underlying biochemical mechanism using peptide pull-down assays followed by mass spectrometry. We identified in bronchial epithelial cells that the adaptor protein SHC1 recognizes tyrosine-phosphorylated CFTR through its phosphotyrosine-binding domain and that the formation of a complex between SHC1 and CFTR is induced at the PM in the presence of activated SYK. The depletion of endogenous SHC1 expression was sufficient to promote an increase in CFTR at the PM of these cells. The results identify a SYK/SHC1 pathway that regulates the PM levels of CFTR channels, contributing to a better understanding of how CFTR-mediated chloride secretion is regulated.
Product Citations: 10
A SYK/SHC1 pathway regulates the amount of CFTR in the plasma membrane.
In Cellular and Molecular Life Sciences : CMLS on 1 December 2020 by Loureiro, C. A., Pinto, F. R., et al.
-
Homo sapiens (Human)
-
Biochemistry and Molecular biology
In Scientific Reports on 22 April 2020 by Thuault, S., Mamelonet, C., et al.
Metastatic progression is the leading cause of mortality in breast cancer. Invasive tumor cells develop invadopodia to travel through basement membranes and the interstitial matrix. Substantial efforts have been made to characterize invadopodia molecular composition. However, their full molecular identity is still missing due to the difficulty in isolating them. To fill this gap, we developed a non-hypothesis driven proteomic approach based on the BioID proximity biotinylation technology, using the invadopodia-specific protein Tks5α fused to the promiscuous biotin ligase BirA* as bait. In invasive breast cancer cells, Tks5α fusion concentrated to invadopodia and selectively biotinylated invadopodia components, in contrast to a fusion which lacked the membrane-targeting PX domain (Tks5β). Biotinylated proteins were isolated by affinity capture and identified by mass spectrometry. We identified known invadopodia components, revealing the pertinence of our strategy. Furthermore, we observed that Tks5 newly identified close neighbors belonged to a biologically relevant network centered on actin cytoskeleton organization. Analysis of Tks5β interactome demonstrated that some partners bound Tks5 before its recruitment to invadopodia. Thus, the present strategy allowed us to identify novel Tks5 partners that were not identified by traditional approaches and could help get a more comprehensive picture of invadopodia molecular landscape.
-
Homo sapiens (Human)
-
Cancer Research
Actin remodeling by Nck regulates endothelial lumen formation.
In Molecular Biology of the Cell on 1 September 2015 by Chaki, S. P., Barhoumi, R., et al.
Multiple angiogenic cues modulate phosphotyrosine signaling to promote vasculogenesis and angiogenesis. Despite its functional and clinical importance, how vascular cells integrate phosphotyrosine-dependent signaling to elicit cytoskeletal changes required for endothelial morphogenesis remains poorly understood. The family of Nck adaptors couples phosphotyrosine signals with actin dynamics and therefore is well positioned to orchestrate cellular processes required in vascular formation and remodeling. Culture of endothelial cells in three-dimensional collagen matrices in the presence of VEGF stimulation was combined with molecular genetics, optical imaging, and biochemistry to show that Nck-dependent actin remodeling promotes endothelial cell elongation and proper organization of VE-cadherin intercellular junctions. Major morphogenetic defects caused by abrogation of Nck signaling included loss of endothelial apical-basal polarity and impaired lumenization. Time-lapse imaging using a Förster resonance energy transfer biosensor, immunostaining with phospho-specific antibodies, and GST pull-down assays showed that Nck determines spatiotemporal patterns of Cdc42/aPKC activation during endothelial morphogenesis. Our results demonstrate that Nck acts as an important hub integrating angiogenic cues with cytoskeletal changes that enable endothelial apical-basal polarization and lumen formation. These findings point to Nck as an emergent target for effective antiangiogenic therapy.
© 2015 Chaki et al. This article is distributed by The American Society for Cell Biology under license from the author(s). Two months after publication it is available to the public under an Attribution–Noncommercial–Share Alike 3.0 Unported Creative Commons License (http://creativecommons.org/licenses/by-nc-sa/3.0).
-
WB
-
Homo sapiens (Human)
-
Cell Biology
Protein interactions of the vesicular glutamate transporter VGLUT1.
In PLoS ONE on 22 October 2014 by Santos, M. S., Foss, S. M., et al.
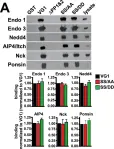
Exocytotic release of glutamate depends upon loading of the neurotransmitter into synaptic vesicles by vesicular glutamate transporters, VGLUTs. The major isoforms, VGLUT1 and 2, exhibit a complementary pattern of expression in synapses of the adult rodent brain that correlates with the probability of release and potential for plasticity. Indeed, expression of different VGLUT protein isoforms confers different properties of release probability. Expression of VGLUT1 or 2 protein also determines the kinetics of synaptic vesicle recycling. To identify molecular determinants that may be related to reported differences in VGLUT trafficking and glutamate release properties, we investigated some of the intrinsic differences between the two isoforms. VGLUT1 and 2 exhibit a high degree of sequence homology, but differ in their N- and C-termini. While the C-termini of VGLUT1 and 2 share a dileucine-like trafficking motif and a proline-, glutamate-, serine-, and threonine-rich PEST domain, only VGLUT1 contains two polyproline domains and a phosphorylation consensus sequence in a region of acidic amino acids. The interaction of a VGLUT1 polyproline domain with the endocytic protein endophilin recruits VGLUT1 to a fast recycling pathway. To identify trans-acting cellular proteins that interact with the distinct motifs found in the C-terminus of VGLUT1, we performed a series of in vitro biochemical screening assays using the region encompassing the polyproline motifs, phosphorylation consensus sites, and PEST domain. We identify interactors that belong to several classes of proteins that modulate cellular function, including actin cytoskeletal adaptors, ubiquitin ligases, and tyrosine kinases. The nature of these interactions suggests novel avenues to investigate the modulation of synaptic vesicle protein recycling.
-
WB
Proteasomal degradation of Nck1 but not Nck2 regulates RhoA activation and actin dynamics.
In Nature Communications on 30 November 2013 by Buvall, L., Rashmi, P., et al.
The ubiquitously expressed adapter proteins Nck1/2 interact with a multitude of effector molecules to regulate diverse cellular functions including cytoskeletal dynamics. Here we show that Nck1, but not Nck2, is a substrate of c-Cbl-mediated ubiquitination. We uncover lysine 178 in Nck1 as the evolutionarily conserved ubiquitin acceptor site. We previously reported that synaptopodin, a proline-rich actin-binding protein, induces stress fibres by blocking the Smurf1-mediated ubiquitination of RhoA. We now find that synaptopodin competes with c-Cbl for binding to Nck1, which prevents the ubiquitination of Nck1 by c-Cbl. Gene silencing of c-Cbl restores Nck1 protein abundance and stress fibres in synaptopodin knockdown cells. Similarly, expression of c-Cbl-resistant Nck1(K178R) or Nck2 containing the SH3 domain 2 of Nck1 restores stress fibres in synaptopodin-depleted podocytes through activation of RhoA signalling. These findings reveal proteasomal regulation as a key factor in the distinct and non-redundant effects of Nck on RhoA-mediated actin dynamics.
-
Cell Biology
In PLoS One on 22 October 2014 by Santos, M. S., Foss, S. M., et al.
Fig.6.A

-
WB
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 1