Abstract In the last few years, probiotics have gained much attention within the medical, pharmaceutical, and food fields, given the health benefits provided by their consumption. They include several lactic acid bacteria (LAB) species, mostly belonging to the genera Lactobacillus, Lactococcus, and Streptococcus. Postbiotics are bioactive compounds (organic acids, short-chain fatty acids, enzymes, and neurotransmitters) produced by bacterial fermentation that exert different health effects. It is well known that probiotics, as health-promoting microorganisms, show different therapeutic properties, including anti-pathogenic, anti-inflammatory, and cholesterol-lowering activities. Recently, accumulating evidence has shown that certain commensal bacteria play protective roles against cancer; thus, anti-carcinogenic activity is one of the most interesting probiotics properties that is currently under investigation. Here, we studied the anticancer properties of postbiotics produced by three different Lactococcus lactis subsp lactis strains isolated from natural whey starter cultures on human glioblastoma cell lines. MTT and Trypan Blue exclusion assays revealed a significant reduction in cell proliferation, and flow cytometry analysis corroborated this data, demonstrating a cell cycle arrest in treated cells. Moreover, other cancer hallmarks, such as wound healing rate closure and migration, were markedly inhibited by postbiotics. On the other hand, primary astrocytes viability and the blood-brain barrier (BBB) integrity were not impaired, suggesting a selective effect of postbiotics on proliferating-undifferentiated cells. This preliminary study highlights, for the first time, the potential anticancer properties of postbiotics from some L. lactis strains on human glioblastoma cell lines.
Product Citations: 93
Antitumor activity of Lactococcus lactis cell-free supernatant on human glioblastoma cell lines
Preprint on Research Square on 29 April 2025 by De Chiara, I., Feola, A., et al.
N-cadherin dynamically regulates pediatric glioma cell migration in complex environments.
In The Journal of Cell Biology on 3 June 2024 by Kim, D., Olson, J. M., et al.
Pediatric high-grade gliomas are highly invasive and essentially incurable. Glioma cells migrate between neurons and glia, along axon tracts, and through extracellular matrix surrounding blood vessels and underlying the pia. Mechanisms that allow adaptation to such complex environments are poorly understood. N-cadherin is highly expressed in pediatric gliomas and associated with shorter survival. We found that intercellular homotypic N-cadherin interactions differentially regulate glioma migration according to the microenvironment, stimulating migration on cultured neurons or astrocytes but inhibiting invasion into reconstituted or astrocyte-deposited extracellular matrix. N-cadherin localizes to filamentous connections between migrating leader cells but to epithelial-like junctions between followers. Leader cells have more surface and recycling N-cadherin, increased YAP1/TAZ signaling, and increased proliferation relative to followers. YAP1/TAZ signaling is dynamically regulated as leaders and followers change position, leading to altered N-cadherin levels and organization. Together, the results suggest that pediatric glioma cells adapt to different microenvironments by regulating N-cadherin dynamics and cell-cell contacts.
© 2024 Kim et al.
-
Cancer Research
-
Cell Biology
Preprint on BioRxiv : the Preprint Server for Biology on 21 August 2023 by Dow, L. P., Surace, S., et al.
Epithelial cell migration is critical in regulating wound healing and tissue development. The epithelial microenvironment is incredibly dynamic, subjected to mechanical cues including cyclic stretch. While cyclic cell stretching platforms have revealed responses of the epithelium such as cell reorientation and gap formation, few studies have investigated the long-term effects of cyclic stretch on cell migration. We measured the migratory response of the epithelium to a range of physiologically relevant frequencies and stretch. We integrated our experimental approach with high-throughput cell segmentation to discover a relationship between changes in cell morphology and migration as a function of cyclic stretch. Our results indicate that lower stretch frequencies (i.e., 0.1 Hz) arrest epithelial migration, accompanied by cell reorientation and high cell shape solidity. We found that this response is also accompanied by increased recruitment of vinculin to cell-cell contacts, and this recruitment is necessary to arrest cell movements. This work demonstrates a critical role for frequency dependence in epithelial response to mechanical stretch. These results confirm the mechanosensitive nature of vinculin within the adherens junction, but independently reveal a novel mechanism of low frequency stress response in supporting epithelial integrity by arresting cell migration.
In Nat Cardiovasc Res on 1 July 2023 by Zhang, Y., Zhang, Y., et al.
Endothelial cells respond to mechanical forces exerted by blood flow. Endothelial cell-cell junctions and the sites of endothelial adhesion to the matrix sense and transmit mechanical forces to the cellular cytoskeleton. Here we show that the scaffold protein AmotL2 connects junctional VE-cadherin and actin filaments to the nuclear lamina. AmotL2 is essential for the formation of radial actin filaments and the alignment of endothelial cells, and, in its absence, nuclear integrity and positioning are altered. Molecular analysis demonstrated that VE-cadherin binds to AmotL2 and actin, resulting in a cascade that transmits extracellular mechanical signals to the nuclear membrane. Furthermore, the endothelial deficit of AmotL2 in mice fed normal diet provoked a pro-inflammatory response and abdominal aortic aneurysms (AAAs). Transcriptome analysis of human AAA samples revealed a negative correlation between AmotL2 and inflammation of the aortic intima. These findings offer insight into the link between junctional mechanotransduction and vascular disease.
© 2023. The Author(s).
-
Immunology and Microbiology
In Mechanisms of Ageing and Development on 1 July 2023 by Pramotton, F. M., Abukar, A., et al.
Aging is the major risk factor for chronic disease development. Cellular senescence is a key mechanism that triggers or contributes to age-related phenotypes and pathologies. The endothelium, a single layer of cells lining the inner surface of a blood vessel, is a critical interface between blood and all tissues. Many studies report a link between endothelial cell senescence, inflammation, and diabetic vascular diseases. Here we identify, using combined advanced AI and machine learning, the Dual Specificity Tyrosine Phosphorylation Regulated Kinase 1B (DYRK1B) protein as a possible senolytic target for senescent endothelial cells. We demonstrate that upon induction of senescence in vitro DYRK1B expression is increased in endothelial cells and localized at adherens junctions where it impairs their proper organization and functions. DYRK1B knock-down or inhibition restores endothelial barrier properties and collective behavior. DYRK1B is therefore a possible target to counteract diabetes-associated vascular diseases linked to endothelial cell senescence.
Copyright © 2023 The Authors. Published by Elsevier B.V. All rights reserved.
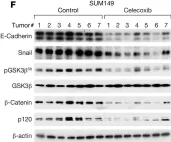
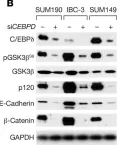
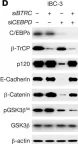
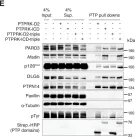
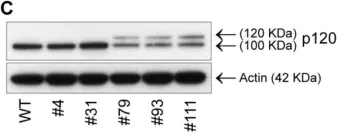
In JCI Insight on 22 March 2023 by Balamurugan, K., Poria, D. K., et al.
Fig.4.F

-
WB
-
Collected and cropped from JCI Insight by CiteAb, provided under a CC-BY license
Image 1 of 21
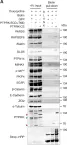
In JCI Insight on 22 March 2023 by Balamurugan, K., Poria, D. K., et al.
Fig.4.A

-
WB
-
Collected and cropped from JCI Insight by CiteAb, provided under a CC-BY license
Image 1 of 21
In JCI Insight on 22 March 2023 by Balamurugan, K., Poria, D. K., et al.
Fig.2.G

-
WB
-
Collected and cropped from JCI Insight by CiteAb, provided under a CC-BY license
Image 1 of 21
In JCI Insight on 22 March 2023 by Balamurugan, K., Poria, D. K., et al.
Fig.1.H

-
WB
-
Collected and cropped from JCI Insight by CiteAb, provided under a CC-BY license
Image 1 of 21
In JCI Insight on 22 March 2023 by Balamurugan, K., Poria, D. K., et al.
Fig.2.B

-
WB
-
Collected and cropped from JCI Insight by CiteAb, provided under a CC-BY license
Image 1 of 21
In JCI Insight on 22 March 2023 by Balamurugan, K., Poria, D. K., et al.
Fig.2.A

-
WB
-
Collected and cropped from JCI Insight by CiteAb, provided under a CC-BY license
Image 1 of 21
In JCI Insight on 22 March 2023 by Balamurugan, K., Poria, D. K., et al.
Fig.2.D

-
WB
-
Collected and cropped from JCI Insight by CiteAb, provided under a CC-BY license
Image 1 of 21
In JCI Insight on 22 March 2023 by Balamurugan, K., Poria, D. K., et al.
Fig.2.F

-
WB
-
Collected and cropped from JCI Insight by CiteAb, provided under a CC-BY license
Image 1 of 21
In JCI Insight on 22 March 2023 by Balamurugan, K., Poria, D. K., et al.
Fig.2.C

-
WB
-
Collected and cropped from JCI Insight by CiteAb, provided under a CC-BY license
Image 1 of 21
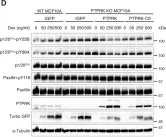
In Pharmaceutics on 16 February 2023 by Lampe, J. B., Desai, P. P., et al.
Fig.7.A

-
WB
-
Collected and cropped from Pharmaceutics by CiteAb, provided under a CC-BY license
Image 1 of 21
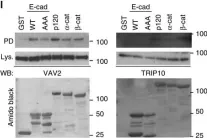
In Elife on 29 March 2019 by Fearnley, G. W., Young, K. A., et al.
Fig.5.E

-
WB
-
Collected and cropped from Elife by CiteAb, provided under a CC-BY license
Image 1 of 21
In Elife on 29 March 2019 by Fearnley, G. W., Young, K. A., et al.
Fig.4.A

-
WB
-
Collected and cropped from Elife by CiteAb, provided under a CC-BY license
Image 1 of 21
In Elife on 29 March 2019 by Fearnley, G. W., Young, K. A., et al.
Fig.6.D

-
WB
-
Collected and cropped from Elife by CiteAb, provided under a CC-BY license
Image 1 of 21
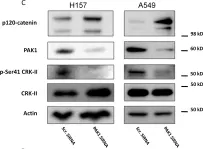
In PLoS One on 30 October 2018 by Falero-Perez, J., Song, Y. S., et al.
Fig.5.B

-
WB
-
Mus musculus (House mouse)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 21
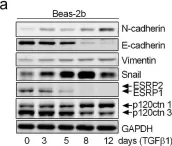
In Sci Rep on 19 October 2018 by Chen, L., He, X., et al.
Fig.4.A

-
WB
-
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 21
In Nat Commun on 6 December 2016 by Erasmus, J. C., Bruche, S., et al.
Fig.7.I

-
WB
-
Homo sapiens (Human)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 21
In PLoS One on 24 December 2014 by Olsen, P. A., Solberg, N. T., et al.
Fig.8.C

-
WB
-
Homo sapiens (Human)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 21
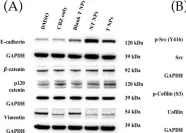
In PLoS One on 1 August 2012 by Rettig, M., Trinidad, K., et al.
Fig.4.C

-
WB
-
Homo sapiens (Human)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 21
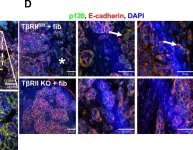
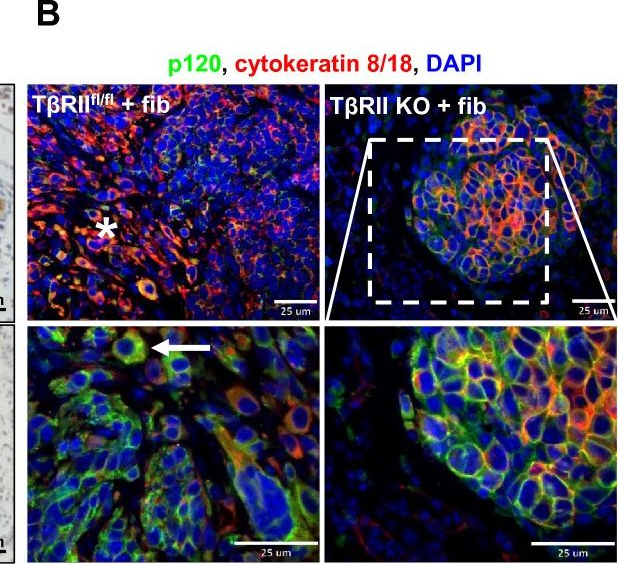
In Breast Cancer Res on 2 July 2012 by Matise, L. A., Palmer, T. D., et al.
Fig.4.D

-
IHC-IF
-
Mus musculus (House mouse)
Collected and cropped from Breast Cancer Res by CiteAb, provided under a CC-BY license
Image 1 of 21
In Breast Cancer Res on 2 July 2012 by Matise, L. A., Palmer, T. D., et al.
Fig.4.C

-
IHC-IF
-
Mus musculus (House mouse)
Collected and cropped from Breast Cancer Res by CiteAb, provided under a CC-BY license
Image 1 of 21
In Breast Cancer Res on 2 July 2012 by Matise, L. A., Palmer, T. D., et al.
Fig.4.B
-
IHC-IF
-
Mus musculus (House mouse)
Collected and cropped from Breast Cancer Res by CiteAb, provided under a CC-BY license
Image 1 of 21