Cadherin family proteins play a central role in epithelial and endothelial cell-cell adhesion. The dynamic regulation of cell adhesion is achieved in part through endocytic membrane trafficking pathways that modulate cadherin cell surface levels. Here, we define the role for various MARCH family ubiquitin ligases in the regulation of cadherin degradation. We find that MARCH2 selectively downregulates VE-cadherin, resulting in loss of adherens junction proteins at cell borders and a loss of endothelial barrier function. Interestingly, N-cadherin is refractory to MARCH ligase expression, demonstrating that different classical cadherin family proteins are differentially regulated by MARCH family ligases. Using chimeric cadherins, we find that the specificity of different MARCH family ligases for different cadherins is conferred by the cadherin transmembrane domain. Further, juxta-membrane lysine residues are required for cadherin degradation by MARCH proteins. These findings expand our understanding of cadherin regulation and highlight a new role for mammalian MARCH family ubiquitin ligases in differentially regulating cadherin turnover.
Copyright: © 2024 Seo et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Product Citations: 71
MARCH family E3 ubiquitin ligases selectively target and degrade cadherin family proteins.
In PLoS ONE on 10 May 2024 by Seo, T., Lowery, A. M., et al.
-
Homo sapiens (Human)
In Developmental Cell on 5 February 2024 by Darrigrand, J. F., Salowka, A., et al.
During organ formation, progenitor cells need to acquire different cell identities and organize themselves into distinct structural units. How these processes are coordinated and how tissue architecture(s) is preserved despite the dramatic cell rearrangements occurring in developing organs remain unclear. Here, we identified cellular rearrangements between acinar and ductal progenitors as a mechanism to drive branching morphogenesis in the pancreas while preserving the integrity of the acinar-ductal functional unit. Using ex vivo and in vivo mouse models, we found that pancreatic ductal cells form clefts by protruding and pulling on the acinar basement membrane, which leads to acini splitting. Newly formed acini remain connected to the bifurcated branches generated by ductal cell rearrangement. Insulin growth factor (IGF)/phosphatidylinositol 3-kinase (PI3K) pathway finely regulates this process by controlling pancreatic ductal tissue fluidity, with a simultaneous impact on branching and cell fate acquisition. Together, our results explain how acinar structure multiplication and branch bifurcation are synchronized during pancreas organogenesis.
Crown Copyright © 2023. Published by Elsevier Inc. All rights reserved.
-
Stem Cells and Developmental Biology
In NPJ Breast Cancer on 13 July 2023 by Yu, J., da Silva, E. M., et al.
This study describes "lobular-like invasive mammary carcinomas" (LLIMCas), a group of low- to intermediate-grade invasive mammary carcinomas with discohesive, diffusely infiltrative cells showing retained circumferential membranous immunoreactivity for both E-cadherin and p120. We analyzed the clinical-pathologic features of 166 LLIMCas compared to 104 classical invasive lobular carcinomas (ILCs) and 100 grade 1 and 2 invasive ductal carcinomas (IDCs). Tumor size and pT stage of LLIMCas were intermediate between IDCs and ILCs, and yet often underestimated on imaging and showed frequent positive margins on the first resection. Despite histomorphologic similarities to classical ILC, the discohesion in LLIMCa was independent of E-cadherin/p120 immunophenotypic alteration. An exploratory, hypothesis-generating analysis of the genomic features of 14 randomly selected LLIMCas and classical ILCs (7 from each category) was performed utilizing an FDA-authorized targeted capture sequencing assay (MSK-IMPACT). None of the seven LLIMCas harbored CDH1 loss-of-function mutations, and none of the CDH1 alterations detected in two of the LLIMCas was pathogenic. In contrast, all seven ILCs harbored CDH1 loss-of-function mutations coupled with the loss of heterozygosity of the CDH1 wild-type allele. Four of the six evaluable LLIMCas were positive for CDH1 promoter methylation, which may partially explain the single-cell infiltrative morphology seen in LLIMCa. Further studies are warranted to better define the molecular basis of the discohesive cellular morphology in LLIMCa. Until more data becomes available, identifying LLIMCas and distinguishing them from typical IDCs and ILCs would be justified. In patients with LLIMCas, preoperative MRI should be entertained to guide surgical management.
© 2023. The Author(s).
-
Cancer Research
Preprint on BioRxiv : the Preprint Server for Biology on 27 January 2023 by Darrigrand, J., Salowka, A., et al.
SUMMARY During organ formation, progenitor cells need to acquire the diversity of cell identities found in the organ as well as organize themselves into distinct structural units. How these processes are coordinated, and how tissue architecture(s) are preserved despite the dramatic cell rearrangements occurring in developing organs remain unclear. Here, we identified cellular rearrangements between acinar and ductal progenitors as a mechanism to drive branching morphogenesis in the pancreas while preserving the integrity of the acinar-ductal functional unit. Using ex vivo and in vivo mouse models, we found that pancreatic ductal cells form clefts by protruding and pulling on the acinar basement membrane, which lead to acini splitting. Newly formed acini remain connected to bifurcated branches generated by ductal cell rearrangement. IGF/PI3K pathway regulates this process by controlling ductal cell fluidity. If components of the pathway are genetically or chemically dysregulated, ductal cell fluidity prevents branching and affects pancreatic cell fates. Hence, our results explain how acinar multiplication and branch bifurcation are synchronized during pancreas organogenesis.
-
Mus musculus (House mouse)
Preprint on BioRxiv : the Preprint Server for Biology on 21 January 2023 by Fitzpatrick, A., Iravani, M., et al.
With a median survival of 4 months, the development of breast cancer leptomeningeal metastasis (BCLM) is devastating for patients. Investigating this often occult disease process is hampered by the difficulty of accessing leptomeningeal tumour material. Here, we utilise cerebrospinal fluid as a source of both cell-free DNA (cfDNA) and disseminated tumour cells, to explore the evolution of BCLM and heterogeneity between leptomeningeal and extracranial sites. CSF cfDNA sequencing detected actionable somatic alterations in 81% (17/21) of BCLM cases. Further genomic characterisation identified frequently altered genes involved in cell adherens junctions and cytoskeleton/chemotaxis pathways. Patient-derived organoids established from CSF tumour cells allowed profiling of this rarely available biological material, in addition to therapeutic testing and generation of in vivo models. Together these data reveal the development of BCLM is associated with the acquisition of a lobular-like breast cancer phenotype, and highlight potential approaches for tackling this hard-to-treat form of metastatic disease.
-
Cancer Research
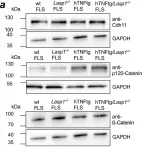
In Nat Commun on 15 June 2021 by Beckmann, D., Römer-Hillmann, A., et al.
Fig.4.A

-
WB
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 10
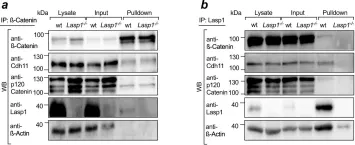
In Nat Commun on 15 June 2021 by Beckmann, D., Römer-Hillmann, A., et al.
Fig.5.A,B

-
WB
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 10
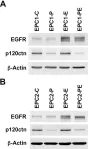
In PLoS One on 29 October 2020 by Landmesser, M. E., Raup-Konsavage, W. M., et al.
Fig.1.A

-
WB
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 10
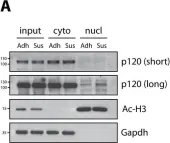
In Dis Model Mech on 1 April 2015 by van de Ven, R. A., Tenhagen, M., et al.
Fig.4.A

-
WB
-
Mus musculus (House mouse)
Collected and cropped from Dis Model Mech by CiteAb, provided under a CC-BY license
Image 1 of 10
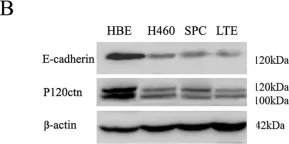
In PLoS One on 23 May 2012 by Yu, J., Miao, Y., et al.
Fig.2.B

-
WB
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 10
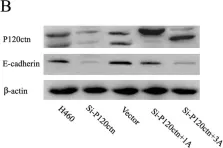
In PLoS One on 23 May 2012 by Yu, J., Miao, Y., et al.
Fig.3.B

-
WB
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 10
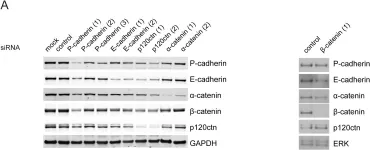
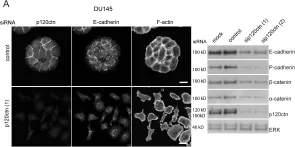
In PLoS One on 30 July 2010 by Kümper, S. & Ridley, A. J.
Fig.5.A

-
WB
-
Homo sapiens (Human)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 10
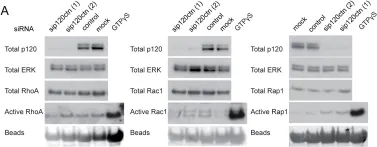
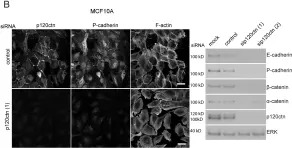
In PLoS One on 30 July 2010 by Kümper, S. & Ridley, A. J.
Fig.4.A

-
WB
-
Homo sapiens (Human)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 10
In PLoS One on 30 July 2010 by Kümper, S. & Ridley, A. J.
Fig.1.A

-
WB
-
Homo sapiens (Human)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 10
In PLoS One on 30 July 2010 by Kümper, S. & Ridley, A. J.
Fig.1.B

-
WB
-
Homo sapiens (Human)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 10