Microvilli are actin-bundle-supported surface protrusions that play essential roles in diverse epithelial functions. To develop our understanding of microvilli biogenesis, we used live imaging to directly visualize protrusion growth at early stages of epithelial differentiation. Time-lapse data revealed that specific factors, including epidermal growth factor pathway substrate 8 (EPS8) and insulin-receptor tyrosine kinase substrate (IRTKS) (also known as BAIAP2L1), appear in diffraction-limited puncta at the cell surface and mark future sites of microvillus growth. New core actin bundles elongate from these puncta in parallel with the arrival of ezrin and subsequent plasma membrane encapsulation. In addition to de novo growth, we also observed that new microvilli emerge from pre-existing protrusions. Moreover, we found that nascent microvilli can also collapse, characterized first by loss of membrane wrapping and ezrin enrichment, followed by a sharp decrease in distal tip EPS8 and IRTKS levels, and ultimately disassembly of the core actin bundle itself. These studies are the first to offer a temporally resolved microvillus growth mechanism and highlight factors that participate in this process; they also provide important insights on the growth of apical specializations that will likely apply to diverse epithelial contexts.
Copyright © 2021 Elsevier Inc. All rights reserved.
Product Citations: 13
Direct visualization of epithelial microvilli biogenesis.
In Current Biology : CB on 21 June 2021 by Gaeta, I. M., Meenderink, L. M., et al.
-
ICC
EPS8 phosphorylation by Src modulates its oncogenic functions.
In British Journal of Cancer on 1 September 2020 by Shahoumi, L. A., Khodadadi, H., et al.
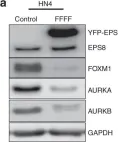
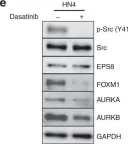
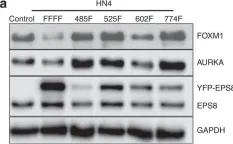
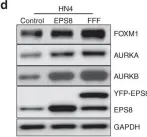
EPS8 is a scaffolding protein that regulates proliferation, actin dynamics and receptor trafficking. Its expression is increased in cancer, enhancing mitogenesis, migration and tumorigenesis. Src phosphorylates EPS8 at four tyrosine residues, although the function is unknown. Here we investigated the pro-oncogenic role of EPS8 tyrosine phosphorylation at Src target sites in HNSCC.
Plasmids expressing EPS8 Src-mediated phosphorylation site mutants (Y485F, Y525F, Y602F, Y774F and all four combined [FFFF]) were expressed in cells containing a normal endogenous level of EPS8. In addition, cells were treated with dasatinib to inhibit Src activity. EPS8 downstream targets were evaluated by western blotting. Wound closure, proliferation, immunofluorescence and tumorgenicity assays were used to investigate the impact of phenylalanine mutations on EPS8 biological functions.
FOXM1, AURKA, and AURKB were decreased in cells expressing FFFF- and Y602F-EPS8 mutants, while cells harbouring the Y485F-, Y525F- and Y774F-EPS8 mutants showed no differences compared to controls. Consistent with this, dasatinib decreased the expression of EPS8 targets. Moreover, Y602F- and FFFF-EPS8 mutants reduced mitogenesis and motility. Strikingly though, FFFF- or Y602F-EPS8 mutants actually promoted tumorigenicity compared with control cells.
Phosphorylation of EPS8 at Y602 is crucial for signalling to the cell cycle and may provide insight to explain reduced efficacy of dasatinib treatment.
-
WB
-
Cancer Research
EPS8 Facilitates Uncoating of Influenza A Virus.
In Cell Reports on 19 November 2019 by Larson, G. P., Tran, V., et al.
All viruses balance interactions between cellular machinery co-opted to support replication and host factors deployed to halt the infection. We use gene correlation analysis to perform an unbiased screen for host factors involved in influenza A virus (FLUAV) infection. Our screen identifies the cellular factor epidermal growth factor receptor pathway substrate 8 (EPS8) as the highest confidence pro-viral candidate. Knockout and overexpression of EPS8 confirm its importance in enhancing FLUAV infection and titers. Loss of EPS8 does not affect virion attachment, uptake, or fusion. Rather, our data show that EPS8 specifically functions during virion uncoating. EPS8 physically associates with incoming virion components, and subsequent nuclear import of released ribonucleoprotein complexes is significantly delayed in the absence of EPS8. Our study identifies EPS8 as a host factor important for uncoating, a crucial step of FLUAV infection during which the interface between the virus and host is still being discovered.
Copyright © 2019 The Author(s). Published by Elsevier Inc. All rights reserved.
-
Immunology and Microbiology
In BMC Cancer on 5 September 2019 by Yu, X., Liang, C., et al.
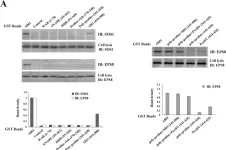
We aimed to develop inhibitory short peptides that can prevent protein interactions of SOS1/EPS8/ABI1 tri-complex, a key component essential for ovarian cancer metastasis.
Plasmids containing various regions of HA-tagged ABI1 were co-transfected into ovarian cancer cells with Flag-tagged SOS1 or Myc-tagged EPS8. Co-immunoprecipitation and GST-pulldown assay were used to identify the regions of ABI1 responsible for SOS1 and EPS8 binding. Inhibitory short peptides of these binding regions were synthesized and modified with HIV-TAT sequence. The blocking effects of the peptides on ABI1-SOS1 or ABI1-EPS8 interactions in vitro and in vivo were determined by GST-pulldown assay. The capability of these short peptides in inhibiting invasion and metastasis of ovarian cancer cell was tested by Matrigel invasion assay and peritoneal metastatic colonization assay.
The formation of endogenous SOS1/EPS8/ABI1 tri-complex was detected in the event of LPA-induced ovarian cancer cell invasion. In the tri-complex, ABI1 acted as a scaffold protein holding together SOS1 and EPS8. The SH3 and poly-proline+PxxDY regions of ABI1 were responsible for SOS1 and EPS8 binding, respectively. Inhibitory short peptides p + p-8 (ppppppppvdyedee) and SH3-3 (ekvvaiydytkdkddelsfmegaii) could block ABI1-SOS1 and ABI1-EPS8 interaction in vitro. TAT-p + p-8 peptide could disrupt ABI1-EPS8 interaction and suppress the invasion and metastasis of ovarian cancer cells in vivo.
TAT-p + p-8 peptide could efficiently disrupt the ABI1-EPS8 interaction, tri-complex formation, and block the invasion and metastasis of ovarian cancer cells.
-
WB
-
Cancer Research
Clarin-2 is essential for hearing by maintaining stereocilia integrity and function.
In EMBO Molecular Medicine on 1 September 2019 by Dunbar, L. A., Patni, P., et al.
Hearing relies on mechanically gated ion channels present in the actin-rich stereocilia bundles at the apical surface of cochlear hair cells. Our knowledge of the mechanisms underlying the formation and maintenance of the sound-receptive structure is limited. Utilizing a large-scale forward genetic screen in mice, genome mapping and gene complementation tests, we identified Clrn2 as a new deafness gene. The Clrn2clarinet/clarinet mice (p.Trp4* mutation) exhibit a progressive, early-onset hearing loss, with no overt retinal deficits. Utilizing data from the UK Biobank study, we could show that CLRN2 is involved in human non-syndromic progressive hearing loss. Our in-depth morphological, molecular and functional investigations establish that while it is not required for initial formation of cochlear sensory hair cell stereocilia bundles, clarin-2 is critical for maintaining normal bundle integrity and functioning. In the differentiating hair bundles, lack of clarin-2 leads to loss of mechano-electrical transduction, followed by selective progressive loss of the transducing stereocilia. Together, our findings demonstrate a key role for clarin-2 in mammalian hearing, providing insights into the interplay between mechano-electrical transduction and stereocilia maintenance.
© 2019 The Authors. Published under the terms of the CC BY 4.0 license.
-
IHC-IF
-
Mus musculus (House mouse)
-
Biochemistry and Molecular biology
In Br J Cancer on 1 September 2020 by Shahoumi, L. A., Khodadadi, H., et al.
Fig.1.A

-
WB
-
Collected and cropped from Br J Cancer by CiteAb, provided under a CC-BY license
Image 1 of 5
In Br J Cancer on 1 September 2020 by Shahoumi, L. A., Khodadadi, H., et al.
Fig.1.E

-
WB
-
Collected and cropped from Br J Cancer by CiteAb, provided under a CC-BY license
Image 1 of 5
In Br J Cancer on 1 September 2020 by Shahoumi, L. A., Khodadadi, H., et al.
Fig.2.A

-
WB
-
Collected and cropped from Br J Cancer by CiteAb, provided under a CC-BY license
Image 1 of 5
In Br J Cancer on 1 September 2020 by Shahoumi, L. A., Khodadadi, H., et al.
Fig.2.D

-
WB
-
Collected and cropped from Br J Cancer by CiteAb, provided under a CC-BY license
Image 1 of 5
In BMC Cancer on 5 September 2019 by Yu, X., Liang, C., et al.
Fig.3.A

-
WB
-
Collected and cropped from BMC Cancer by CiteAb, provided under a CC-BY license
Image 1 of 5