The RAS-MAPK system plays an important role in regulating various cellular processes, including growth, differentiation, apoptosis, and transformation. Dysregulation of this system has been implicated in genetic diseases and cancers affecting diverse tissues. To better understand the regulation of this system, we employed information flow analysis based on transfer entropy (TE) between the activation dynamics of two key elements in cells stimulated with EGF: SOS, a guanine nucleotide exchanger for the small GTPase RAS, and RAF, a RAS effector serine/threonine kinase. TE analysis allows for model-free assessment of the timing, direction, and strength of the information flow regulating the system response. We detected significant amounts of TE in both directions between SOS and RAF, indicating feedback regulation. Importantly, the amount of TE did not simply follow the input dose or the intensity of the causal reaction, demonstrating the uniqueness of TE. TE analysis proposed regulatory networks containing multiple tracks and feedback loops and revealed temporal switching in the reaction pathway primarily responsible for reaction control. This proposal was confirmed by the effects of an MEK inhibitor on TE. Furthermore, TE analysis identified the functional disorder of a SOS mutation associated with Noonan syndrome, a human genetic disease, of which the pathogenic mechanism has not been precisely known yet. TE assessment holds significant promise as a model-free analysis method of reaction networks in molecular pharmacology and pathology.
© 2025, Umeki et al.
Product Citations: 54
Evaluation of information flows in the RAS-MAPK system using transfer entropy measurements.
In eLife on 6 March 2025 by Umeki, N., Kabashima, Y., et al.
Inhibition and degradation of NRAS with a pan-NRAS monobody.
In Oncogene on 1 November 2024 by Whaby, M., Ketavarapu, G., et al.
The RAS family GTPases are the most frequently mutated oncogene family in human cancers. Activating mutations in either of the three RAS isoforms (HRAS, KRAS, or NRAS) are found in nearly 20% of all human tumors with NRAS mutated in ~25% of melanomas. Despite remarkable advancements in therapies targeted against mutant KRAS, NRAS-specific pharmacologics are lacking. Thus, development of inhibitors of NRAS would address a critical unmet need to treating primary tumors harboring NRAS mutations as well as BRAF-mutant melanomas, which frequently develop resistance to clinically approved BRAF inhibitors through NRAS mutation. Building upon our previous studies with the monobody NS1 that recognizes HRAS and KRAS but not NRAS, here we report the development of a monobody that specifically binds to both GDP and GTP-bound states of NRAS and inhibits NRAS-mediated signaling in a mutation-agnostic manner. Further, this monobody can be formatted into a genetically encoded NRAS-specific degrader. Our study highlights the feasibility of developing NRAS selective inhibitors for therapeutic efforts.
© 2024. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
-
Homo sapiens (Human)
-
Cancer Research
In Biomolecules on 4 December 2023 by Kirkbride, J. A., Nilsson, G. Y., et al.
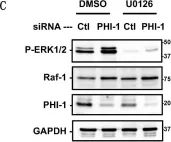
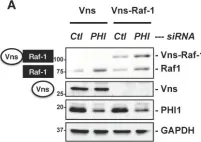
Raf-1, a multifunctional kinase, regulates various cellular processes, including cell proliferation, apoptosis, and migration, by phosphorylating MAPK/ERK kinase and interacting with specific kinases. Cellular Raf-1 activity is intricately regulated through pathways involving the binding of regulatory proteins, direct phosphorylation, and the ubiquitin-proteasome axis. In this study, we demonstrate that PHI-1, an endogenous inhibitor of protein phosphatase-1 (PP1), plays a pivotal role in modulating Raf-1 proteostasis within cells. Knocking down endogenous PHI-1 in HEK293 cells using siRNA resulted in increased cell proliferation and reduced apoptosis. This heightened cell proliferation was accompanied by a 15-fold increase in ERK1/2 phosphorylation. Importantly, the observed ERK1/2 hyperphosphorylation was attributable to an upregulation of Raf-1 expression, rather than an increase in Ras levels, Raf-1 Ser338 phosphorylation, or B-Raf levels. The elevated Raf-1 expression, stemming from PHI-1 knockdown, enhanced EGF-induced ERK1/2 phosphorylation through MEK. Moreover, PHI-1 knockdown significantly contributed to Raf-1 protein stability without affecting Raf-1 mRNA levels. Conversely, ectopic PHI-1 expression suppressed Raf-1 protein levels in a manner that correlated with PHI-1's inhibitory potency. Inhibiting PP1 to mimic PHI-1's function using tautomycin led to a reduction in Raf-1 expression. In summary, our findings highlight that the PHI-1-PP1 signaling axis selectively governs Raf-1 proteostasis and cell survival signals.
-
WB
-
Homo sapiens (Human)
-
Biochemistry and Molecular biology
-
Cancer Research
In Pharmacology on 29 November 2023 by Marunouchi, T., Matsumura, K., et al.
There is still no effective treatment for heart failure with preserved left ventricular ejection fraction (HFpEF), and therapies to improve prognosis are urgently needed. Clinical studies in patients with HFpEF have shown that statins and HMG-CoA reductase inhibitors may reduce their mortality rate. However, the mechanisms underlying the effects of statins on HFpEF remain unknown. In the present study, we examined whether simvastatin administration inhibits the development of cardiac fibrosis in HFpEF model mice. We further examined the contribution of the Smad and mitogen-activated protein (MAP) kinase pathways to the transforming growth factor-β (TGF-β) signaling pathway in the development of HFpEF.
HFpEF animals were prepared by feeding C57BL/6 N mice a high-fat diet and providing water containing N[w]-nitro-l-arginine methyl ester hydrochloride (l-NAME) for 15 weeks. Simvastatin (30 mg/kg/day) or vehicle was administered orally daily during the experimental period. Cardiac function was measured by echocardiography, and cardiac fibrosis was evaluated by Masson's trichrome staining. Changes in the TGF-β signaling proteins in myocardial tissue were examined by Western blotting.
A high-fat diet and l-NAME solution load induced cardiac diastolic dysfunction with cardiac fibrosis. Simvastatin treatment markedly attenuated cardiac fibrosis and reduced cardiac diastolic dysfunction. In addition, simvastatin prevented the increase in phosphorylation levels of Smad (Smad2 and Smad3) and MAPK (c-Raf, Erk1/2) pathway proteins downstream of the TGF-β receptor in cardiac tissue.
Our present study demonstrated that simvastatin attenuated diastolic dysfunction by reducing cardiac fibrosis in HFpEF hearts. Furthermore, our findings suggest that the mechanisms by which simvastatin attenuates HFpEF development involve, at least in part, inhibition of the TGF-β signaling pathway, which is activated in the HFpEF heart.
© 2023 S. Karger AG, Basel.
-
WB
-
Mus musculus (House mouse)
-
Cardiovascular biology
-
Pharmacology
In Cell Death & Disease on 12 January 2023 by Pedrazza, L., Martinez-Martinez, A., et al.
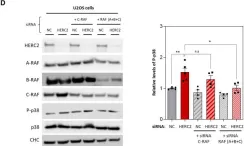
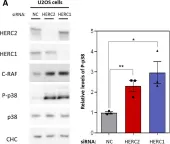
Bone remodeling is a continuous process between bone-forming osteoblasts and bone-resorbing osteoclasts, with any imbalance resulting in metabolic bone disease, including osteopenia. The HERC1 gene encodes an E3 ubiquitin ligase that affects cellular processes by regulating the ubiquitination of target proteins, such as C-RAF. Of interest, an association exists between biallelic pathogenic sequence variants in the HERC1 gene and the neurodevelopmental disorder MDFPMR syndrome (macrocephaly, dysmorphic facies, and psychomotor retardation). Most pathogenic variants cause loss of HERC1 function, and the affected individuals present with features related to altered bone homeostasis. Herc1-knockout mice offer an excellent model in which to study the role of HERC1 in bone remodeling and to understand its role in disease. In this study, we show that HERC1 regulates osteoblastogenesis and osteoclastogenesis, proving that its depletion increases gene expression of osteoblastic makers during the osteogenic differentiation of mesenchymal stem cells. During this process, HERC1 deficiency increases the levels of C-RAF and of phosphorylated ERK and p38. The Herc1-knockout adult mice developed imbalanced bone homeostasis that presented as osteopenia in both sexes of the adult mice. By contrast, only young female knockout mice had osteopenia and increased number of osteoclasts, with the changes associated with reductions in testosterone and dihydrotestosterone levels. Finally, osteocytes isolated from knockout mice showed a higher expression of osteocytic genes and an increase in the Rankl/Opg ratio, indicating a relevant cell-autonomous role of HERC1 when regulating the transcriptional program of bone formation. Overall, these findings present HERC1 as a modulator of bone homeostasis and highlight potential therapeutic targets for individuals affected by pathological HERC1 variants.
© 2023. The Author(s).
-
WB
-
Biochemistry and Molecular biology
-
Cell Biology
In Biomolecules on 4 December 2023 by Kirkbride, J. A., Nilsson, G. Y., et al.
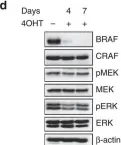
Fig.3.C

-
WB
-
Homo sapiens (Human)
Collected and cropped from Biomolecules by CiteAb, provided under a CC-BY license
Image 1 of 26
In Biomolecules on 4 December 2023 by Kirkbride, J. A., Nilsson, G. Y., et al.
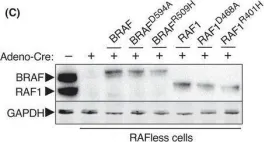
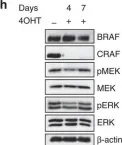
Fig.4.A

-
WB
-
Homo sapiens (Human)
Collected and cropped from Biomolecules by CiteAb, provided under a CC-BY license
Image 1 of 26
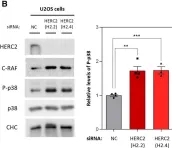
In Int J Mol Sci on 1 January 2023 by Abuasaker, B., Garrido, E., et al.
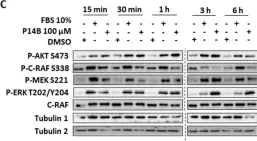
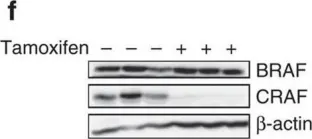
Fig.2.C

-
WB
-
Collected and cropped from Int J Mol Sci by CiteAb, provided under a CC-BY license
Image 1 of 26
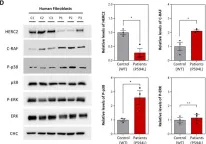
In Cell Mol Life Sci on 14 October 2022 by Sala-Gaston, J., Pedrazza, L., et al.
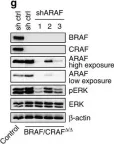
Fig.6.A

-
WB
-
Collected and cropped from Cell Mol Life Sci by CiteAb, provided under a CC-BY license
Image 1 of 26
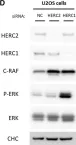
In Cell Mol Life Sci on 14 October 2022 by Sala-Gaston, J., Pedrazza, L., et al.
Fig.4.D

-
WB
-
Collected and cropped from Cell Mol Life Sci by CiteAb, provided under a CC-BY license
Image 1 of 26
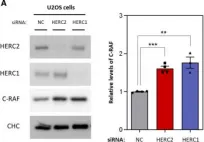
In Cell Mol Life Sci on 14 October 2022 by Sala-Gaston, J., Pedrazza, L., et al.
Fig.3.B

-
WB
-
Collected and cropped from Cell Mol Life Sci by CiteAb, provided under a CC-BY license
Image 1 of 26
In Cell Mol Life Sci on 14 October 2022 by Sala-Gaston, J., Pedrazza, L., et al.
Fig.1.A

-
WB
-
Collected and cropped from Cell Mol Life Sci by CiteAb, provided under a CC-BY license
Image 1 of 26
In Cell Mol Life Sci on 14 October 2022 by Sala-Gaston, J., Pedrazza, L., et al.
Fig.1.D

-
WB
-
Collected and cropped from Cell Mol Life Sci by CiteAb, provided under a CC-BY license
Image 1 of 26
In Cell Mol Life Sci on 14 October 2022 by Sala-Gaston, J., Pedrazza, L., et al.
Fig.2.D

-
WB
-
Collected and cropped from Cell Mol Life Sci by CiteAb, provided under a CC-BY license
Image 1 of 26
In Cell Mol Life Sci on 14 October 2022 by Sala-Gaston, J., Pedrazza, L., et al.
Fig.2.A

-
WB
-
Collected and cropped from Cell Mol Life Sci by CiteAb, provided under a CC-BY license
Image 1 of 26
In Cell Mol Life Sci on 14 October 2022 by Sala-Gaston, J., Pedrazza, L., et al.
Fig.3.A

-
WB
-
Collected and cropped from Cell Mol Life Sci by CiteAb, provided under a CC-BY license
Image 1 of 26
In Mol Oncol on 1 September 2022 by Paniagua, G., Jacob, H. K. C., et al.
Fig.5.C

-
WB
-
Collected and cropped from Mol Oncol by CiteAb, provided under a CC-BY license
Image 1 of 26
In Mol Oncol on 1 September 2022 by Paniagua, G., Jacob, H. K. C., et al.
Fig.4.D

-
WB
-
Collected and cropped from Mol Oncol by CiteAb, provided under a CC-BY license
Image 1 of 26
In Mol Oncol on 1 September 2022 by Paniagua, G., Jacob, H. K. C., et al.
Fig.4.A

-
WB
-
Collected and cropped from Mol Oncol by CiteAb, provided under a CC-BY license
Image 1 of 26
In Nat Commun on 12 May 2017 by Dorard, C., Estrada, C., et al.
Fig.3.D

-
WB
-
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 26
In Nat Commun on 12 May 2017 by Dorard, C., Estrada, C., et al.
Fig.3.H

-
WB
-
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 26
In Nat Commun on 12 May 2017 by Dorard, C., Estrada, C., et al.
Fig.3.F

-
WB
-
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 26
In Nat Commun on 12 May 2017 by Dorard, C., Estrada, C., et al.
Fig.6.G

-
WB
-
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 26
In Nat Commun on 12 May 2017 by Dorard, C., Estrada, C., et al.
Fig.7.A

-
WB
-
Homo sapiens (Human)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 26
In Nat Commun on 12 May 2017 by Dorard, C., Estrada, C., et al.
Fig.6.E

-
WB
-
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 26
In Nat Commun on 12 May 2017 by Dorard, C., Estrada, C., et al.
Fig.5.A

-
WB
-
Homo sapiens (Human)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 26
In Nat Commun on 12 May 2017 by Dorard, C., Estrada, C., et al.
Fig.6.B

-
WB
-
Homo sapiens (Human)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 26
In PLoS One on 23 March 2012 by Yu, Z., Sato, S., et al.
Fig.9.C

-
WB
-
Homo sapiens (Human)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 26
In PLoS One on 23 March 2012 by Yu, Z., Sato, S., et al.
Fig.9.B

-
WB
-
Homo sapiens (Human)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 26
In PLoS One on 23 March 2012 by Yu, Z., Sato, S., et al.
Fig.3.E

-
WB
-
Homo sapiens (Human)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 26