VE-cadherin is a major component of endothelial adherens junctions and pivotal to the regulation of vascular barrier integrity. Whereas two phosphorylation sites of VE-cadherin (Y685 and Y731) are known to be relevant for the regulation of endothelial junctions in vivo, several others were suggested to be relevant based on in vitro studies. Here, we analyze for two of these, serine 665 (S665) and tyrosine 658 (Y658), whether they are relevant for the induction of vascular permeability in vivo. To this end, we generated and characterized two point-mutated VE-cadherin knock-in mouse lines where either S665 was replaced by valine (S665V) or Y658 by phenylalanine (Y658F). We found that the induction of vascular permeability by histamine or VEGF in the skin was clearly reduced in S665V mice, whereas Y658F mice showed a normal increase of permeability. In line with this, we found that histamine-induced endocytosis was impaired for the VE-cadherin-S665V mutant, but not for the Y658F mutant. Comparing the regulation of VE-cadherin phosphorylation at S665, Y658 and Y685, we found that only phosphorylation of S665 and Y685 were strongly induced by inflammatory mediators, while phosphorylation of Y658 increased weakly. Interestingly, phosphorylation of S665 and Y685 occurred with different kinetics, but independent of each other. Collectively, our results demonstrate that Y658 is irrelevant for vascular leak formation in the context of several tested inflammatory mediators and establish S665 of VE-cadherin as an important phosphorylation site regulating the induction of endothelial permeability in vivo.
© 2025. The Author(s).
Product Citations: 30
In Cellular and Molecular Life Sciences : CMLS on 5 June 2025 by Holtermann, L., Rivera-Galdos, R., et al.
-
Biochemistry and Molecular biology
-
Immunology and Microbiology
Preprint on Research Square on 15 April 2025 by Holtermann, L., Rivera-Galdos, R., et al.
Abstract VEcadherin is a major component of endothelial adherens junctions and pivotal to the regulation of vascular barrier integrity. Whereas two phosphorylation sites of VEcadherin (Y685 and Y731) are known to be relevant for the regulation of endothelial junctions in vivo, several others were suggested to be relevant based on in vitro studies. Here, we analyze for two of these, serine 665 (S665) and tyrosine 658 (Y658), whether they are relevant for the induction of vascular permeability in vivo. To this end, we generated and characterized two point-mutated VEcadherin knockin mouse lines where either S665 was replaced by valine (S665V) or Y658 by phenylalanine (Y658F). We found that the induction of vascular permeability by histamine or VEGF in the skin was clearly reduced in S665V mice, whereas Y658F mice showed a normal increase of permeability. In line with this, we found that histamine-induced endocytosis was impaired for the VEcadherin-S665V mutant, but not for the Y658F mutant. Comparing the regulation of VEcadherin phosphorylation at S665, Y658 and Y685, we found that only phosphorylation of S665 and Y685 were strongly induced by inflammatory mediators, while phosphorylation of Y658 increased weakly. Interestingly, phosphorylation of S665 and Y685 occurred with different kinetics, but independent of each other. Collectively, our results demonstrate that Y658 is irrelevant for vascular leak formation under inflammatory conditions and establish S665 of VEcadherin as an important phosphorylation site regulating the induction of endothelial permeability in vivo.
-
Immunology and Microbiology
Ubiquitination of VE-cadherin regulates inflammation-induced vascular permeability in vivo.
In EMBO Reports on 1 September 2024 by Wilkens, M., Holtermann, L., et al.
VE-cadherin is a major component of the cell adhesion machinery which provides integrity and plasticity of the barrier function of endothelial junctions. Here, we analyze whether ubiquitination of VE-cadherin is involved in the regulation of the endothelial barrier in inflammation in vivo. We show that histamine and thrombin stimulate ubiquitination of VE-cadherin in HUVEC, which is completely blocked if the two lysine residues K626 and K633 are replaced by arginine. Similarly, these mutations block histamine-induced endocytosis of VE-cadherin. We describe two knock-in mouse lines with endogenous VE-cadherin being replaced by either a VE-cadherin K626/633R or a VE-cadherin KallR mutant, where all seven lysine residues are mutated. Mutant mice are viable, healthy and fertile with normal expression levels of junctional VE-cadherin. Histamine- or LPS-induced vascular permeability in the skin or lung of both of these mutant mice are clearly and similarly reduced in comparison to WT mice. Additionally, we detect a role of K626/633 for lysosomal targeting. Collectively, our findings identify ubiquitination of VE-cadherin as important for the induction of vascular permeability in the inflamed skin and lung.
© 2024. The Author(s).
-
Immunology and Microbiology
In Nature Communications on 20 September 2023 by Mapunda, J. A., Pareja, J., et al.
Meninges cover the surface of the brain and spinal cord and contribute to protection and immune surveillance of the central nervous system (CNS). How the meningeal layers establish CNS compartments with different accessibility to immune cells and immune mediators is, however, not well understood. Here, using 2-photon imaging in female transgenic reporter mice, we describe VE-cadherin at intercellular junctions of arachnoid and pia mater cells that form the leptomeninges and border the subarachnoid space (SAS) filled with cerebrospinal fluid (CSF). VE-cadherin expression also marked a layer of Prox1+ cells located within the arachnoid beneath and separate from E-cadherin+ arachnoid barrier cells. In vivo imaging of the spinal cord and brain in female VE-cadherin-GFP reporter mice allowed for direct observation of accessibility of CSF derived tracers and T cells into the SAS bordered by the arachnoid and pia mater during health and neuroinflammation, and detection of volume changes of the SAS during CNS pathology. Together, the findings identified VE-cadherin as an informative landmark for in vivo imaging of the leptomeninges that can be used to visualize the borders of the SAS and thus potential barrier properties of the leptomeninges in controlling access of immune mediators and immune cells into the CNS during health and neuroinflammation.
© 2023. Springer Nature Limited.
-
Immunology and Microbiology
In Cellular and Molecular Life Sciences : CMLS on 24 January 2022 by Thölmann, S., Seebach, J., et al.
Junctional adhesion molecule (JAM)-A is a cell adhesion receptor localized at epithelial cell-cell contacts with enrichment at the tight junctions. Its role during cell-cell contact formation and epithelial barrier formation has intensively been studied. In contrast, its role during collective cell migration is largely unexplored. Here, we show that JAM-A regulates collective cell migration of polarized epithelial cells. Depletion of JAM-A in MDCK cells enhances the motility of singly migrating cells but reduces cell motility of cells embedded in a collective by impairing the dynamics of cryptic lamellipodia formation. This activity of JAM-A is observed in cells grown on laminin and collagen-I but not on fibronectin or vitronectin. Accordingly, we find that JAM-A exists in a complex with the laminin- and collagen-I-binding α3β1 integrin. We also find that JAM-A interacts with tetraspanins CD151 and CD9, which both interact with α3β1 integrin and regulate α3β1 integrin activity in different contexts. Mapping experiments indicate that JAM-A associates with α3β1 integrin and tetraspanins CD151 and CD9 through its extracellular domain. Similar to depletion of JAM-A, depletion of either α3β1 integrin or tetraspanins CD151 and CD9 in MDCK cells slows down collective cell migration. Our findings suggest that JAM-A exists with α3β1 integrin and tetraspanins CD151 and CD9 in a functional complex to regulate collective cell migration of polarized epithelial cells.
© 2022. The Author(s).
-
IF
-
Biochemistry and Molecular biology
In Respir Res on 28 June 2013 by Wang, Q., Wang, Y., et al.
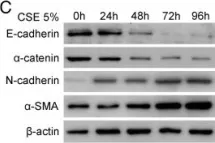
Fig.4.C

-
WB
-
Homo sapiens (Human)
Collected and cropped from Respir Res by CiteAb, provided under a CC-BY license
Image 1 of 2
In Respir Res on 28 June 2013 by Wang, Q., Wang, Y., et al.
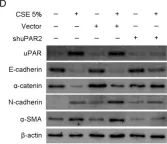
Fig.5.D

-
WB
-
Homo sapiens (Human)
Collected and cropped from Respir Res by CiteAb, provided under a CC-BY license
Image 1 of 2