In AD, amyloid pathology (A) precedes progressive development of tau pathology (T) and neurodegeneration (N), with the latter (T/N) processes associated with symptom progression. Recent anti-amyloid beta (Aβ) clinical trials raise hope but indicate the need for multi-targeted therapies, to effectively halt clinical AD and ATN pathology progression. APOE-related putative protective mutations (including APOE3Christchurch, RELN-COLBOS) were recently identified in case reports with exceptionally high resilience to autosomal dominant AD. In these cases, Nature provided proof of concept for halting autosomal dominant AD and ATN progression in humans, despite a high amyloid load, and pointing to the APOE pathway as a potential target. This is further supported by the recent identification of APOE4 homozygosity as genetic AD. Here we studied the role of APOE in a preclinical model that robustly mimics amyloid-facilitated (A) tau pathology (T) and subsequent neurodegeneration (N), denoted as ATN model, generated by crossing 5xFAD (F +) and TauP301S (T +) mice. We show that APOE deficiency, markedly inhibited progression to tau pathology and tau-induced neurodegeneration in this ATN model, despite a high Aβ load, reminiscent of the high resilience ADAD case reports. Further study identified, despite increased Aβ load (W02 stained), a significant decrease in compacted, dense core plaques stained by ThioS in APOE deficient ATN mice. Furthermore, single-cell RNA sequencing (scRNA-seq) showed a crucial role of APOE in microglial conversion beyond homeostatic microglia to reactive and disease associated microglia (DAM) in this ATN preclinical model. Microglial elimination significantly decreased amyloid-driven tau pathology, in the presence of APOE, but not in APOE deficient mice. Together the data demonstrate that APOE deficiency inhibits amyloid-driven tau pathology and subsequent neurodegeneration, by pleiotropic effects including prevention of dense core plaque formation and halting conversion of homeostatic microglia. We here present a model recapitulating inhibition of amyloid-facilitated tau pathology by APOE deficiency despite high Aβ load, important for understanding the role of APOE, and APOE-dependent processes in ATN progression and its therapeutic exploitation in AD.
© 2025. The Author(s).
Product Citations: 126
In Molecular Psychiatry on 30 April 2025 by Vanherle, S., Janssen, A., et al.
-
Neuroscience
-
Pathology
In Journal of Extracellular Vesicles on 1 April 2025 by Lee, Y., Kim, H., et al.
Extracellular vesicles (EVs) and secretory factors play crucial roles in intercellular communication, but the molecular mechanisms and dynamics governing their interplay in human pluripotent stem cells (hPSCs) are poorly understood. Here, we demonstrate that hPSC-secreted milk fat globule-EGF factor 8 (MFGE-8) is the principal corona protein at the periphery of EVs, playing an essential role in controlling hPSC stemness. MFGE-8 depletion reduced EV-mediated self-renewal and survival in hPSC cultures. MFGE-8 in the EV corona bound to integrin αvβ5 expressed in the peripheral zone of hPSC colonies. It activated cyclin D1 and dynamin-1 via the AKT/GSK3β axis, promoting the growth of hPSCs and facilitating the endocytosis of EVs. Internalization of EVs alleviated oxidative stress and cell death by transporting redox and stress response proteins that increased GSH levels. Our findings demonstrate the critical role of the extracellular association of MFGE-8 and EVs in modulating the self-renewal and survival of hPSCs.
© 2025 The Author(s). Journal of Extracellular Vesicles published by Wiley Periodicals, LLC on behalf of the International Society for Extracellular Vesicles.
-
WB
-
Stem Cells and Developmental Biology
In Scientific Reports on 25 November 2024 by Rashid, A., Fung, H. L., et al.
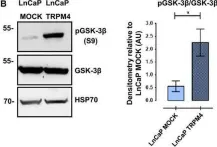
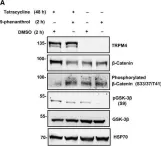
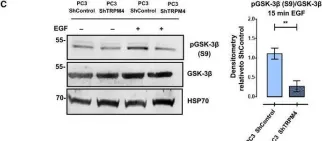
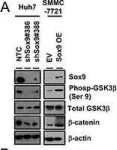
Prostate cancer (PCa) is the most common malignancy diagnosed in men. The purpose of this study was to report the molecular pathways of Homo sapiens solute carrier family 4 member 4 (SLC4A4) in the progression of PCa. Here, we report our findings from clinical specimens of prostatic acinar adenocarcinoma collected from patients. We found that low-grade prostate cancers have higher SLC4A4 expression. We investigated the role of SLC4A4 and the signaling mechanism underlying its role in modulating the PCa progression. Firstly, we reported the SLC4A4/GSK-3β/β-catenin signaling axis, which regulates the clonogenic potential, invasiveness, and metastasis. In this, we found reduced phosphorylation of GSK at serine 21 of α and serine 9 of the β subunit in shSLC4A4 cells of PCa, which ultimately relieved the activity of GSK-3β. This activated GSK-3β phosphorylates β-catenin at Ser33/37 with a subsequently reduced β-catenin level in PCa cells. Our functional analysis revealed that SLC4A4 knockdown retards tumor growth and lowers invasion and migration potential. Secondly, we investigated the SLC4A4/RB axis, which acts to drive cell proliferation. SLC4A4 knockdown decreases the interaction between these molecules with hypophosphorylation of RB protein and cell cycle arrest. Likewise, transcriptome sequencing using the SLC4A4 knockdown in DU145 cells regulates differentiated expressed genes and multiple metabolic pathways. Our results suggest that SLC4A4 may serve as a potential therapeutic target for prostate cancer patients in the future.
© 2024. The Author(s).
-
WB
-
Cancer Research
Preprint on BioRxiv : the Preprint Server for Biology on 5 February 2024 by Silva, P., Hernandez, N., et al.
Tumor hypoxia has been associated with cancer progression, angiogenesis, and metastasis via modifications in the release and cargo composition of extracellular vesicles secreted by tumor cells. Indeed, hypoxic extracellular vesicles are known to trigger a variety of angiogenic responses via different mechanisms. We recently showed that hypoxia promotes endosomal signaling in tumor cells via HIF-1α-dependent induction of the guanine exchange factor ALS2, which activates Rab5, leading to downstream events involved in cell migration and invasion. Since Rab5-dependent signaling is required for endothelial cell migration and angiogenesis, we explored the possibility that hypoxia promotes the release of small extracellular vesicles containing ALS2, which in turn activate Rab5 in recipient endothelial cells leading to pro-angiogenic properties. In doing so, we found that hypoxia promoted ALS2 expression and incorporation as cargo within small extracellular vesicles, leading to subsequent transfer to recipient endothelial cells, promoting cell migration, tube formation and downstream Rab5 activation. Consequently, ALS2-containing small extracellular vesicles increased early endosome size and number in recipient endothelial cells, which was followed by subsequent sequestration of components of the β-catenin destruction complex within endosomal compartments, leading to stabilization and nuclear localization of β-catenin. These events converged in the expression of β-catenin target genes involved in angiogenesis. Knockdown of ALS2 in donor-tumor cells, which precluded its incorporation into small extracellular vesicles, prevented Rab5-downstream events and endothelial cell responses, which depended on Rab5 activity and guanine exchange factor activity of ALS2. These findings indicate that vesicular ALS2, secreted in hypoxia, promotes endothelial cell events leading to angiogenesis. Finally, these events might explain how tumor angiogenesis proceeds in hypoxic conditions.
-
Cancer Research
In Nature Communications on 31 January 2024 by Jeon, Y. G., Nahmgoong, H., et al.
In mammals, brown adipose tissue (BAT) and inguinal white adipose tissue (iWAT) execute sequential thermogenesis to maintain body temperature during cold stimuli. BAT rapidly generates heat through brown adipocyte activation, and further iWAT gradually stimulates beige fat cell differentiation upon prolonged cold challenges. However, fat depot-specific regulatory mechanisms for thermogenic activation of two fat depots are poorly understood. Here, we demonstrate that E3 ubiquitin ligase RNF20 orchestrates adipose thermogenesis with BAT- and iWAT-specific substrates. Upon cold stimuli, BAT RNF20 is rapidly downregulated, resulting in GABPα protein elevation by controlling protein stability, which stimulates thermogenic gene expression. Accordingly, BAT-specific Rnf20 suppression potentiates BAT thermogenic activity via GABPα upregulation. Moreover, upon prolonged cold stimuli, iWAT RNF20 is gradually upregulated to promote de novo beige adipogenesis. Mechanistically, iWAT RNF20 mediates NCoR1 protein degradation, rather than GABPα, to activate PPARγ. Together, current findings propose fat depot-specific regulatory mechanisms for temporal activation of adipose thermogenesis.
© 2024. The Author(s).
In Elife on 13 October 2023 by Broca-Brisson, L., Harati, R., et al.
Fig.6.A

-
WB
-
Collected and cropped from Elife by CiteAb, provided under a CC-BY license
Image 1 of 16
In PLoS One on 8 January 2020 by Alcaraz, E., Vilardell, J., et al.
Fig.3.B

-
WB
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 16
In PLoS One on 8 January 2020 by Alcaraz, E., Vilardell, J., et al.
Fig.3.C

-
WB
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 16
In Cell Commun Signal on 7 November 2018 by Betten, R., Scharner, B., et al.
Fig.4.B

-
WB
-
Collected and cropped from Cell Commun Signal by CiteAb, provided under a CC-BY license
Image 1 of 16
In Mol Oncol on 1 February 2018 by Sagredo, A. I., Sagredo, E. A., et al.
Fig.4.A

-
WB
-
Collected and cropped from Mol Oncol by CiteAb, provided under a CC-BY license
Image 1 of 16
In Mol Oncol on 1 February 2018 by Sagredo, A. I., Sagredo, E. A., et al.
Fig.4.B

-
WB
-
Collected and cropped from Mol Oncol by CiteAb, provided under a CC-BY license
Image 1 of 16
In Mol Oncol on 1 February 2018 by Sagredo, A. I., Sagredo, E. A., et al.
Fig.5.A

-
WB
-
Collected and cropped from Mol Oncol by CiteAb, provided under a CC-BY license
Image 1 of 16
In Mol Oncol on 1 February 2018 by Sagredo, A. I., Sagredo, E. A., et al.
Fig.7.C

-
WB
-
Collected and cropped from Mol Oncol by CiteAb, provided under a CC-BY license
Image 1 of 16
In Oncotarget on 24 October 2017 by Zhu, Y., Zhou, Q., et al.
Fig.5.E

-
WB
-
Collected and cropped from Oncotarget by CiteAb, provided under a CC-BY license
Image 1 of 16
In Oncotarget on 17 May 2016 by Leung, C. O., Mak, W. N., et al.
Fig.5.A

-
WB
-
Collected and cropped from Oncotarget by CiteAb, provided under a CC-BY license
Image 1 of 16
In PLoS One on 25 July 2015 by Cymerman, I. A., Gozdz, A., et al.
Fig.2.A

-
WB
-
Mus musculus (House mouse)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 16
In PLoS One on 25 July 2015 by Cymerman, I. A., Gozdz, A., et al.
Fig.3.A

-
ICC-IF
-
Mus musculus (House mouse)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 16
In Nat Commun on 3 July 2015 by Kim, A. Y., Park, Y. J., et al.
Fig.6.D

-
WB
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 16
In Nat Commun on 3 July 2015 by Kim, A. Y., Park, Y. J., et al.
Fig.6.E

-
WB
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 16
In Mol Neurodegener on 23 November 2014 by Hu, X., Li, X., et al.
Fig.3.E

-
WB
-
Drosophila melanogaster (Fruit fly)
Collected and cropped from Mol Neurodegener by CiteAb, provided under a CC-BY license
Image 1 of 16
In J Hematol Oncol on 10 December 2013 by Phillip, C. J., Zaman, S., et al.
Fig.7.C

-
WB
-
Collected and cropped from J Hematol Oncol by CiteAb, provided under a CC-BY license
Image 1 of 16