κB-Ras/RalGAP complexes limit the activity of Ral GTPases, which function in EGFR/Ras signaling. RalGAP expression is down-regulated in pancreatic cancer; however, the role of RalGAP and Ral GTPases in tumor development in vivo remained unclear. Here, we show that pancreatic RalGAPβ deficiency alone is sufficient to induce inflammation and neoplasia in vivo. We identify that this phenotype is triggered by disturbance of the secretory pathway and polarized exocytosis in acinar cells, demonstrating that RalGAP complexes uphold spatial control of Ral activity. We furthermore show that RALGAPβ deficiency results in defective primary cilium assembly, a process required for efficient acinar regeneration upon inflammation. Only primary cilium formation depends on κB-Ras proteins, suggesting that κB-Ras proteins are not essential for all RalGAP complex-controlled processes. In combination with an oncogenic KRAS G12D mutation, RalGAPβ deficiency leads to a dramatic shortening of tumor latency and median survival. Our results highlight an important role of RalGAP/Ral signaling in upholding acinar cell identity and preventing pancreatic cancer development.
© 2025 Apken et al.
Product Citations: 32
RalGAP complexes control secretion and primary cilia in pancreatic disease.
In Life Science Alliance on 1 August 2025 by Apken, L. H., Barz, H., et al.
-
WB
-
Mus musculus (House mouse)
Hepatic glycogen directly regulates gluconeogenesis through an AMPK/CRTC2 axis in mice.
In The Journal of Clinical Investigation on 2 June 2025 by Zhang, B., Johnson, M. M., et al.
Glycogenolysis and gluconeogenesis ensure sufficient hepatic glucose production during energy shortages. Here, we report that hepatic glycogen levels control the phosphorylation of a transcriptional coactivator to determine the amplitude of gluconeogenesis. Decreased liver glycogen during fasting promotes gluconeogenic gene expression, while feeding-induced glycogen accumulation suppresses it. Liver-specific deletion of the glycogen scaffolding protein, protein targeting to glycogen (PTG), reduces glycogen levels, increases the expression of gluconeogenic genes, and promotes glucose production in primary hepatocytes. In contrast, liver glycogen phosphorylase (PYGL) knockdown or inhibition increases glycogen levels and represses gluconeogenic gene expression. These changes in hepatic glycogen levels are sensed by AMP-activated protein kinase (AMPK). AMPK activity is increased when glycogen levels decline, resulting in the phosphorylation and stabilization of CREB-regulated transcriptional coactivator 2 (CRTC2), which is crucial for the full activation of the cAMP-responsive transcriptional factor CREB. High glycogen allosterically inhibits AMPK, leading to CRTC2 degradation and reduced CREB transcriptional activity. Hepatocytes with low glycogen levels or high AMPK activity show higher CRTC2 protein levels, priming the cell for gluconeogenesis through transcriptional regulation. Thus, glycogen plays a regulatory role in controlling hepatic glucose metabolism through the glycogen/AMPK/CRTC2 signaling axis, safeguarding efficient glucose output during fasting and suppressing it during feeding.
Mitolnc controls cardiac BCAA metabolism and heart hypertrophy by allosteric activation of BCKDH.
In Nucleic Acids Research on 24 June 2024 by Weiss, M., Hettrich, S., et al.
Enzyme activity is determined by various different mechanisms, including posttranslational modifications and allosteric regulation. Allosteric activators are often metabolites but other molecules serve similar functions. So far, examples of long non-coding RNAs (lncRNAs) acting as allosteric activators of enzyme activity are missing. Here, we describe the function of mitolnc in cardiomyocytes, a nuclear encoded long non-coding RNA, located in mitochondria and directly interacting with the branched-chain ketoacid dehydrogenase (BCKDH) complex to increase its activity. The BCKDH complex is critical for branched-chain amino acid catabolism (BCAAs). Inactivation of mitolnc in mice reduces BCKDH complex activity, resulting in accumulation of BCAAs in the heart and cardiac hypertrophy via enhanced mTOR signaling. We found that mitolnc allosterically activates the BCKDH complex, independent of phosphorylation. Mitolnc-mediated regulation of the BCKDH complex constitutes an important additional layer to regulate the BCKDH complex in a tissue-specific manner, evading direct coupling of BCAA metabolism to ACLY-dependent lipogenesis.
© The Author(s) 2024. Published by Oxford University Press on behalf of Nucleic Acids Research.
-
WB
-
Biochemistry and Molecular biology
-
Cardiovascular biology
-
Cell Biology
Shigella flexneri subverts host polarized exocytosis to enhance cell-to-cell spread.
In Molecular Microbiology on 1 November 2021 by Herath, T. U. B., Roy, A., et al.
Shigella flexneri is a gram-negative bacterial pathogen that causes dysentery. Critical for disease is the ability of Shigella to use an actin-based motility (ABM) process to spread between cells of the colonic epithelium. ABM transports bacteria to the periphery of host cells, allowing the formation of plasma membrane protrusions that mediate spread to adjacent cells. Here we demonstrate that efficient protrusion formation and cell-to-cell spread of Shigella involves bacterial stimulation of host polarized exocytosis. Using an exocytic probe, we found that exocytosis is locally upregulated in bacterial protrusions in a manner that depends on the Shigella type III secretion system. Experiments involving RNA interference (RNAi) indicate that efficient bacterial protrusion formation and spread require the exocyst, a mammalian multi-protein complex known to mediate polarized exocytosis. In addition, the exocyst component Exo70 and the exocyst regulator RalA were recruited to Shigella protrusions, suggesting that bacteria manipulate exocyst function. Importantly, RNAi-mediated depletion of exocyst proteins or RalA reduced the frequency of protrusion formation and also the lengths of protrusions, demonstrating that the exocyst controls both the initiation and elongation of protrusions. Collectively, our results reveal that Shigella co-opts the exocyst complex to disseminate efficiently in host cell monolayers.
© 2021 John Wiley & Sons Ltd.
-
Immunology and Microbiology
In International Journal of Molecular Sciences on 5 October 2021 by Hasegawa, K., Takenaka, N., et al.
Insulin stimulates glucose uptake in adipose tissue and skeletal muscle by inducing plasma membrane translocation of the glucose transporter GLUT4. Although the small GTPase Rac1 is a key regulator downstream of phosphoinositide 3-kinase (PI3K) and the protein kinase Akt2 in skeletal muscle, it remains unclear whether Rac1 also regulates glucose uptake in white adipocytes. Herein, we investigated the physiological role of Rac1 in white adipocytes by employing adipocyte-specific rac1 knockout (adipo-rac1-KO) mice. Subcutaneous and epididymal white adipose tissues (WATs) in adipo-rac1-KO mice showed significant reductions in size and weight. Actually, white adipocytes lacking Rac1 were smaller than controls. Insulin-stimulated glucose uptake and GLUT4 translocation were abrogated in rac1-KO white adipocytes. On the other hand, GLUT4 translocation was augmented by constitutively activated PI3K or Akt2 in control, but not in rac1-KO, white adipocytes. Similarly, to skeletal muscle, the involvement of another small GTPase RalA downstream of Rac1 was demonstrated. In addition, mRNA levels of various lipogenic enzymes were down-regulated in rac1-KO white adipocytes. Collectively, these results suggest that Rac1 is implicated in insulin-dependent glucose uptake and lipogenesis in white adipocytes, and reduced insulin responsiveness due to the deficiency of Rac1 may be a likely explanation for atrophy of WATs.
-
WB
-
Mus musculus (House mouse)
-
Endocrinology and Physiology
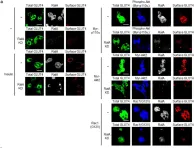
In Int J Mol Sci on 31 October 2019 by Takenaka, N., Nakao, M., et al.
Fig.7.A

-
ICC-IF
-
Collected and cropped from Int J Mol Sci by CiteAb, provided under a CC-BY license
Image 1 of 4
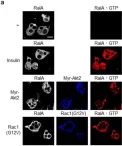
In Int J Mol Sci on 31 October 2019 by Takenaka, N., Nakao, M., et al.
Fig.8.A

-
ICC-IF
-
Collected and cropped from Int J Mol Sci by CiteAb, provided under a CC-BY license
Image 1 of 4
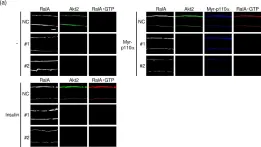
In PLoS One on 9 February 2019 by Takenaka, N., Araki, N., et al.
Fig.4.A

-
ICC-IF
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 4
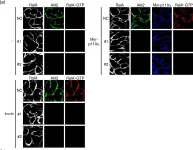
In PLoS One on 9 February 2019 by Takenaka, N., Araki, N., et al.
Fig.5.A

-
ICC-IF
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 4