ABSTRACT Tribbles homolog 2 (TRIB2) is one of three members of the Tribbles family of pseudo serine/threonine kinases. It acts as an oncogene whose expression is correlated with tumor stage and therapy response in melanoma. While metastatic melanoma, the most aggressive form of skin cancer now has two effective treatment options, namely MAPK pathway and immune checkpoint inhibitors, the majority of patients primarily fail to respond or acquire secondary resistance. Hence, innovative approaches to identify disease-relevant, druggable targets to remove the roadblock of therapy resistance are urgently needed. TRIB2 has been implicated in conferring resistance to various anti-cancer therapies suggesting TRIB2 as a therapeutic target for resistant tumors. This study explores the pharmacological targeting of TRIB2, revealing several independent routes of pharmacological manipulations. TRIB2 is a short-lived protein stabilized by inhibition of the PI3K/AKT pathway. Conversely, inhibitors of BRAF, MEK and ERK significantly decrease TRIB2 expression by a mechanism that involves transcription. Strikingly, increasing concentrations of the kinase inhibitor PIK75 effectively eliminate TRIB2 in melanoma cells surpassing its PI3K inhibitory activity. Additionally, Polo-Like Kinases (PLKs) inhibitors significantly reduce TRIB2 protein expression and stability. We demonstrate that inhibition or silencing of PLK2 leads to a decrease in TRIB2 levels. Overall, we identify three distinct classes of compounds that efficiently eliminate the oncogenic TRIB2 protein from melanoma cells based on different molecular mechanisms and exhibiting 40 to 200 times greater potency than the previously reported afatinib.
Product Citations: 6
Preprint on BioRxiv : the Preprint Server for Biology on 21 July 2024 by Mayoral-Varo, V., Orea-Soufi, A., et al.
-
WB
-
Homo sapiens (Human)
-
Cancer Research
In IScience on 20 October 2023 by Postiglione, A. E., Adams, L. L., et al.
Extracellular signal-regulated kinases 1 and 2 (ERK1/2) are dysregulated in many pervasive diseases. Recently, we discovered that ERK1/2 is oxidized by signal-generated hydrogen peroxide in various cell types. Since the putative sites of oxidation lie within or near ERK1/2's ligand-binding surfaces, we investigated how oxidation of ERK2 regulates interactions with the model substrates Sub-D and Sub-F. These studies revealed that ERK2 undergoes sulfenylation at C159 on its D-recruitment site surface and that this modification modulates ERK2 activity differentially between substrates. Integrated biochemical, computational, and mutational analyses suggest a plausible mechanism for peroxide-dependent changes in ERK2-substrate interactions. Interestingly, oxidation decreased ERK2's affinity for some D-site ligands while increasing its affinity for others. Finally, oxidation by signal-generated peroxide enhanced ERK1/2's ability to phosphorylate ribosomal S6 kinase A1 (RSK1) in HeLa cells. Together, these studies lay the foundation for examining crosstalk between redox- and phosphorylation-dependent signaling at the level of kinase-substrate selection.
© 2023 The Authors.
In Biomolecules on 22 July 2020 by Ramaniuk, O., Klímová, Z., et al.
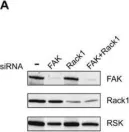
Cells attaching to the extracellular matrix spontaneously acquire front-rear polarity. This self-organization process comprises spatial activation of polarity signaling networks and the establishment of a protruding cell front and a non-protruding cell rear. Cell polarization also involves the reorganization of cell mass, notably the nucleus that is positioned at the cell rear. It remains unclear, however, how these processes are regulated. Here, using coherence-controlled holographic microscopy (CCHM) for non-invasive live-cell quantitative phase imaging (QPI), we examined the role of the focal adhesion kinase (FAK) and its interacting partner Rack1 in dry mass distribution in spreading Rat2 fibroblasts. We found that FAK-depleted cells adopt an elongated, bipolar phenotype with a high central body mass that gradually decreases toward the ends of the elongated processes. Further characterization of spreading cells showed that FAK-depleted cells are incapable of forming a stable rear; rather, they form two distally positioned protruding regions. Continuous protrusions at opposite sides results in an elongated cell shape. In contrast, Rack1-depleted cells are round and large with the cell mass sharply dropping from the nuclear area towards the basal side. We propose that FAK and Rack1 act differently yet coordinately to establish front-rear polarity in spreading cells.
-
WB
-
Biochemistry and Molecular biology
In Frontiers in Oncology on 7 March 2019 by Moriguchi, M., Watanabe, T., et al.
Primary effusion lymphoma (PEL) is defined as a rare subtype of non-Hodgkin's B-cell lymphoma which is caused by Kaposi's sarcoma-associated herpesvirus (KSHV) in immunosuppressed patients. PEL is an aggressive lymphoma and is frequently resistant to conventional chemotherapies. Therefore, it is critical to investigate novel therapeutic options for PEL. Capsaicin is a pungent component of chili pepper and possesses unique pharmacological effects, such as pain relief, anti-microbial and anti-cancer properties. Here, we demonstrate that capsaicin markedly inhibited the growth of KSHV latently infected PEL cells by inhibiting ERK, p38 MAPK and expression hIL-6, which are known to contribute to PEL growth and survival. The underlying mechanism of action by capsaicin was through the inhibition of ERK and p38 MAPK phosphorylation and signaling that affected hIL-6 expression. As a result, capsaicin induced apoptosis in PEL cells in a caspase-9 dependent manner. In line with these results, ERK (U0126) and p38 MAPK (SB203580) specific signaling inhibitors suppressed hIL-6 expression and attenuated cell growth in PEL cells. Furthermore, the addition of hIL-6 neutralizing antibody to culture medium suppressed the growth of PEL cells. We also demonstrate that capsaicin suppressed PEL cell growth in the absence of nascent viral replication. Finally, we confirmed ex vivo treatment of capsaicin attenuated PEL development in SCID mice. Taken together, capsaicin could represent a lead compound for PEL therapy without the risk of de novo KSHV infection.
-
WB
-
Cancer Research
Use of inhibitors in the study of MAP kinases.
In Methods in Molecular Biology (Clifton, N.J.) on 3 September 2010 by Burkhard, K. & Shapiro, P.
The mitogen-activated protein (MAP) kinases are ubiquitous intracellular signaling proteins that respond to a variety of extracellular signals and regulate most cellular functions including proliferation, apoptosis, migration, differentiation, and secretion. The four major MAP kinase family members, which include the ERK1/2, JNK, p38, and ERK5 proteins, coordinate cellular responses by phosphorylating and regulating the activity of dozens of substrate proteins involved in transcription, translation, and changes in cellular architecture. Uncontrolled activation of the MAP kinases has been implicated in the initiation and progression of a variety of cancers and inflammatory disorders. As such, the ability to manipulate the activity of MAP kinase proteins with specific pharmacological inhibitors has received much attention as research tools for understanding basic mechanisms of cellular functions and for clinical tools to treat diseases. A variety of pharmacological inhibitors have been developed to selectively block MAP kinases directly or indirectly through targeting upstream regulators. This chapter will provide an overview of some of the current inhibitors that target MAP kinase signaling pathways and provide methodology on how to use selective MAP kinase inhibitors and immunoblotting techniques to monitor and quantify phosphorylation of MAP kinase substrates.
-
WB
-
Homo sapiens (Human)
-
Biochemistry and Molecular biology
In Biomolecules on 22 July 2020 by Ramaniuk, O., Klímová, Z., et al.
Fig.2.A

-
WB
-
Collected and cropped from Biomolecules by CiteAb, provided under a CC-BY license
Image 1 of 1