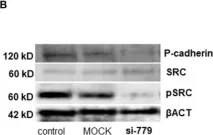
ABSTRACT Thousands of regulatory noncoding RNAs (ncRNAs) have been annotated; however, their functions in gene regulation and contributions to cancer formation remain poorly understood. To gain a better understanding of the influence of ncRNAs on gene regulation during melanoma progression, we mapped the landscape of ncRNAs in melanocytes and melanoma cells. Nearly half of deregulated genes in melanoma are ncRNAs, with antisense RNAs (asRNAs) comprising a large portion of deregulated ncRNAs. CDH3-AS1 , the most significantly downregulated asRNA, overlaps the CDH3 gene, which encodes P-cadherin, a transmembrane glycoprotein involved in cell adhesion that was also reduced in melanoma. Overexpression of CDH3-AS1 increased cell aggregation and reduced xenograft tumor growth, mimicking the tumor-suppressive effects of CDH3 . CDH3-AS1 interacted with CDH3 mRNA and enhanced P-cadherin protein levels. Interestingly, secondary structures at the CDH3 5’ end regulated P-cadherin translation, and ribosome profiling revealed that CDH3-AS1 promotes ribosome occupancy at the CDH3 mRNA. Notably, ribosome occupancy was generally increased in mRNAs having cognate asRNA that are complementary to the 5’UTR. Taken together, this study revealed the CDH3-AS1 -mediated enhancement of P-cadherin translation, underscoring the broader potential of asRNAs as regulators of protein-coding genes and their role in diseases like melanoma.
Product Citations: 24
CDH3-AS1 antisense RNA enhances P-cadherin translation and acts as a tumor suppressor in melanoma
Preprint on BioRxiv : the Preprint Server for Biology on 26 December 2024 by Chadourne, M., Griffith, C., et al.
-
Biochemistry and Molecular biology
-
Cancer Research
-
Genetics
In Modern Pathology : An Official Journal of the United States and Canadian Academy of Pathology, Inc on 1 July 2024 by De Schepper, M., Koorman, T., et al.
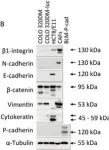
Invasive lobular carcinoma (ILC) is the second most frequent type of breast cancer (BC) and its peculiar morphology is mainly driven by inactivation of CDH1, the gene coding for E-cadherin cell adhesion protein. ILC-specific therapeutic and disease-monitoring approaches are gaining momentum in the clinic, increasing the importance of accurate ILC diagnosis. Several essential and desirable morphologic diagnostic criteria are currently defined by the World Health Organization, the routine use of immunohistochemistry (IHC) for E-cadherin is not recommended. Disagreement in the diagnosis of ILC has been repeatedly reported, but interpathologist agreement increases with the use of E-cadherin IHC. In this study, we aimed to harmonize the pathological diagnosis of ILC by comparing 5 commonly used E-cadherin antibody clones (NCH-38, EP700Y, Clone 36, NCL-L-E-cad [Clone 36B5], and ECH-6). We determined their biochemical specificity for the E-cadherin protein and IHC staining performance according to type and location of mutation on the CDH1 gene. Western blot analysis on mouse cell lines with conditional E-cadherin expression revealed a reduced specificity of EP700Y and NCL-L-E-cad for E-cadherin, with cross-reactivity of Clone 36 to P-cadherin. The use of IHC improved interpathologist agreement for ILC, lobular carcinoma in situ, and atypical lobular hyperplasia. The E-cadherin IHC staining pattern was associated with variant allele frequency and likelihood of nonsense-mediated RNA decay but not with the type or position of CDH1 mutations. Based on these results, we recommend the indication for E-cadherin staining, choice of antibodies, and their interpretation to standardize ILC diagnosis in current pathology practice.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
-
Cancer Research
-
Pathology
Preprint on BioRxiv : the Preprint Server for Biology on 3 June 2024 by Ma, J., To, S. K. Y., et al.
Peritoneal metastasis exacerbates the prognosis of ovarian cancer patients. Adhesion of cancer cells to mesothelium is a rate-limiting prerequisite for this process. How metastatic cells sense and respond to the dynamic biomechanical microenvironment at the mesothelial niche to initiate metastatic lesions remains unclear. Here, the study demonstrates that highly metastatic (HM), but not non-metastatic (NM) ovarian cancer cells, selectively activate the peritoneal mesothelium. Atomic force microscopy reveals that HM cells exert increased adhesive force on mesothelial cells via P-cadherin, a cell-cell adhesion molecule abundant in late-stage tumors. Transcriptomic and molecular analyses show that mechanical induction of P-cadherin enhances lipogenic gene expression and lipid content in HM cells by SREBP1. P-cadherin activation does not affect lipogenic activity but induces glycolysis in the interacting mesothelium. Targeted lipidomic analysis reveals that lactate produced by the glycolytic mesothelium facilitates metastatic outgrowth as a direct substrate for de novo lipogenesis. Inhibiting lactate shuttling via nanodelivery of siRNA targeting P-cadherin or MCT1/4 transporters significantly suppresses metastasis in mice. The association of high fatty acid synthase in patient metastatic samples and increased P-cadherin expression supports enhanced de novo lipogenesis in the metastatic niche. The study reveals P-cadherin-mediated mechano-metabolic coupling as a promising target to restrain peritoneal metastasis.
-
WB
-
Biochemistry and Molecular biology
-
Cancer Research
-
Cell Biology
In The Journal of Pathology on 1 June 2024 by Monster, J. L., Kemp, L. J., et al.
Loss of the cell-cell adhesion protein E-cadherin underlies the development of diffuse-type gastric cancer (DGC), which is characterized by the gradual accumulation of tumor cells originating from the gastric epithelium in the surrounding stroma. How E-cadherin deficiency drives DGC formation remains elusive. Therefore, we investigated the consequences of E-cadherin loss on gastric epithelial organization utilizing a human gastric organoid model and histological analyses of early-stage DGC lesions. E-cadherin depletion from gastric organoids recapitulates DGC initiation, with progressive loss of a single-layered architecture and detachment of individual cells. We found that E-cadherin deficiency in gastric epithelia does not lead to a general loss of epithelial cohesion but disrupts the spindle orientation machinery. This leads to a loss of planar cell division orientation and, consequently, daughter cells are positioned outside of the gastric epithelial layer. Although basally delaminated cells fail to detach and instead reintegrate into the epithelium, apically mispositioned daughter cells can trigger the gradual loss of the single-layered epithelial architecture. This impaired architecture hampers reintegration of mispositioned daughter cells and enables basally delaminated cells to disseminate into the surrounding matrix. Taken together, our findings describe how E-cadherin deficiency disrupts gastric epithelial architecture through displacement of dividing cells and provide new insights in the onset of DGC. © 2024 The Authors. The Journal of Pathology published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.
© 2024 The Authors. The Journal of Pathology published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.
-
Cancer Research
-
Pathology
Identification of new head and neck squamous cell carcinoma molecular imaging targets.
In Oral Oncology on 1 April 2024 by van Schaik, J. E., van der Vegt, B., et al.
Intraoperative fluorescence imaging (FI) of head and neck squamous cell carcinoma (HNSCC) is performed to identify tumour-positive surgical margins, currently using epidermal growth factor receptor (EGFR) as imaging target. EGFR, not exclusively present in HNSCC, may result in non-specific tracer accumulation in normal tissues. We aimed to identify new potential HNSCC FI targets.
Publicly available transcriptomic data were collected, and a biostatistical method (Transcriptional Adaptation to Copy Number Alterations (TACNA)-profiling) was applied. TACNA-profiling captures downstream effects of CNAs on mRNA levels, which may translate to protein-level overexpression. Overexpressed genes were identified by comparing HNSCC versus healthy oral mucosa. Potential targets, selected based on overexpression and plasma membrane expression, were immunohistochemically stained. Expression was compared to EGFR on paired biopsies of HNSCC, adjacent macroscopically suspicious mucosa, and healthy mucosa.
TACNA-profiling was applied on 111 healthy oral mucosa and 410 HNSCC samples, comparing expression levels of 19,635 genes. The newly identified targets were glucose transporter-1 (GLUT-1), placental cadherin (P-cadherin), monocarboxylate transporter-1 (MCT-1), and neural/glial antigen-2 (NG2), and were evaluated by IHC on samples of 31 patients. GLUT-1 was expressed in 100 % (median; range: 60-100 %) of tumour cells, P-cadherin in 100 % (50-100 %), EGFR in 70 % (0-100 %), MCT-1 in 30 % (0-100 %), and NG2 in 10 % (0-70 %). GLUT-1 and P-cadherin showed higher expression than EGFR (p < 0.001 and p = 0.015).
The immunohistochemical confirmation of TACNA-profiling results showed significantly higher GLUT-1 and P-cadherin expression than EGFR, warranting further investigation as HNSCC FI targets.
Copyright © 2024 The Authors. Published by Elsevier Ltd.. All rights reserved.
-
Homo sapiens (Human)
-
Cancer Research
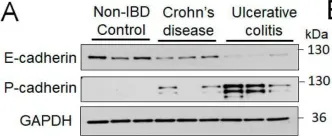
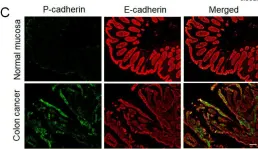
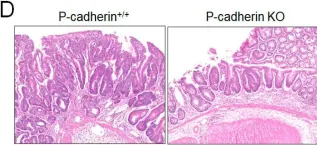
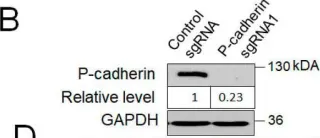
In Cells on 27 April 2022 by Naydenov, N. G., Lechuga, S., et al.
Fig.1.A

-
WB
-
Collected and cropped from Cells by CiteAb, provided under a CC-BY license
Image 1 of 8
In Cells on 27 April 2022 by Naydenov, N. G., Lechuga, S., et al.
Fig.1.C

-
IHC-IF
-
Collected and cropped from Cells by CiteAb, provided under a CC-BY license
Image 1 of 8
In Cells on 27 April 2022 by Naydenov, N. G., Lechuga, S., et al.
Fig.3.D

-
IHC
-
Collected and cropped from Cells by CiteAb, provided under a CC-BY license
Image 1 of 8
In Cells on 27 April 2022 by Naydenov, N. G., Lechuga, S., et al.
Fig.4.B

-
WB
-
Collected and cropped from Cells by CiteAb, provided under a CC-BY license
Image 1 of 8
In Cells on 27 April 2022 by Naydenov, N. G., Lechuga, S., et al.
Fig.4.A

-
WB
-
Collected and cropped from Cells by CiteAb, provided under a CC-BY license
Image 1 of 8
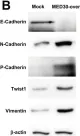
In Oncotarget on 26 April 2019 by Timmermans-Sprang, E., Collin, R., et al.
Fig.5.B

-
WB
-
Collected and cropped from Oncotarget by CiteAb, provided under a CC-BY license
Image 1 of 8
In Oncotarget on 15 November 2016 by Tommelein, J., Gremonprez, F., et al.
Fig.2.B

-
WB
-
Collected and cropped from Oncotarget by CiteAb, provided under a CC-BY license
Image 1 of 8
In PLoS One on 26 June 2015 by Lee, Y. J., Han, M. E., et al.
Fig.6.B

-
WB
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 8