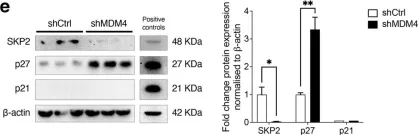
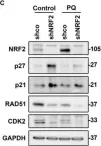
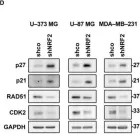
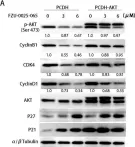
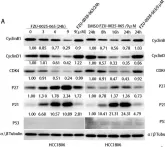
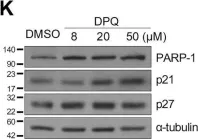
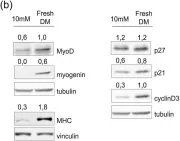
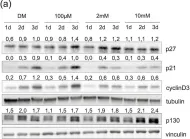
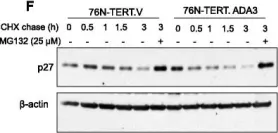
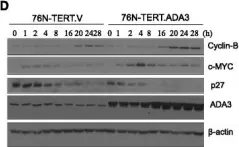
KRIBB11, a heat shock factor1 (HSF1) inhibitor, sensitizes cancer cells to several anticancer drugs. We have previously demonstrated that KRIBB11 alone induced the apoptosis of A172 glioblastoma cells. However, the molecular basis of its anticancer activity remains unclear. Hence, we aimed to examine the alterations in cell cycle regulators and the relevance of HSF1 activity following KRIBB11 treatment in A172 cells.
The expression levels of p21, p27, and p53 were determined using western blotting or real-time PCR. Alterations in p27 levels were induced using small interfering RNA and retroviral transfection. SKP2 degradation was analyzed through a cycloheximide chase assay.
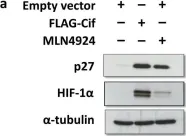
p21 and p27 exhibited opposite expression profiles in A172 cells following KRIBB11 treatment, with p21 accumulating and p27 decreasing, respectively. Further experiments revealed that p21 induction could be attributed to HSF1-dependent p53 accumulation, which is responsible for cell cycle arrest and apoptosis. In contrast, p27 reduction was not reproduced by HSF1 silencing; however, further suppression of p27 accelerated poly (ADP-ribose) polymerase cleavage by KRIBB11 treatment, which was partially reversed by p27 overexpression. Thus, the reduction in p27 levels by KRIBB11 appeared favorable for apoptosis, suggesting that p27 functions as an oncogene in A172 cells. Subsequently, we demonstrated that the decrease in p27 levels following KRIBB11 exposure was mediated by the accumulation of the SKP2 protein, accompanied by a reduction in Cdh1 ubiquitin ligase.
KRIBB11 induces apoptotic cell death in A172 cells through two axes: HSF1-dependent p53/p21 accumulation and Cdh1/SKP2-dependent reduction of p27.
Copyright © 2025 International Institute of Anticancer Research (Dr. George J. Delinasios), All rights reserved.
Product Citations: 286
In Cancer Genomics Proteomics on 26 April 2025 by Yoo, K., Yun, H. H., et al.
-
Cancer Research
Mitoregulin Promotes Cell Cycle Progression in Non-Small Cell Lung Cancer Cells.
In International Journal of Molecular Sciences on 24 February 2025 by Stein, C. S., Linzer, C. R., et al.
Mitoregulin (MTLN) is a 56-amino-acid mitochondrial microprotein known to modulate mitochondrial energetics. MTLN gene expression is elevated broadly across most cancers and has been proposed as a prognostic biomarker for non-small cell lung cancer (NSCLC). In addition, lower MTLN expression in lung adenocarcinoma (LUAD) correlates with significantly improved patient survival. In our studies, we have found that MTLN silencing in A549 NSCLC cells slowed proliferation and, in accordance with this, we observed the following: (1) increased proportion of cells in the G1 phase of cell cycle; (2) protein changes consistent with G1 arrest (e.g., reduced levels and/or reduced phosphorylation of ERK, MYC, CDK2, and RB, and elevated p27Kip1); (3) reduction in clonogenic cell survival and; (4) lower steady-state cytosolic and mitochondrial H2O2 levels as indicated by use of the roGFP2-Orp1 redox sensor. Conflicting with G1 arrest, we observed a boost in cyclin D1 abundance. We also tested MTLN silencing in combination with buthionine sulfoximine (BSO) and auranofin (AF), drugs that inhibit GSH synthesis and thioredoxin reductase, respectively, to elevate the reactive oxygen species (ROS) amount to a toxic range. Interestingly, clonogenic survival after drug treatment was greater for MTLN-silenced cultures versus the control cultures. Lower H2O2 output and reduced vulnerability to ROS damage due to G1 status may have jointly contributed to the partial BSO + AF resistance. Overall, our results provide evidence that MTLN fosters H2O2 signaling to propel G1/S transition and suggest MTLN silencing as a therapeutic strategy to limit NSCLC growth.
-
WB
-
Cancer Research
Netrin1 patterns the dorsal spinal cord through modulation of Bmp signaling.
In Cell Reports on 26 November 2024 by Alvarez, S., Gupta, S., et al.
We have identified an unexpected role for netrin1, a canonical axonal guidance cue, as a suppressor of bone morphogenetic protein (Bmp) signaling in the developing dorsal spinal cord. Using a combination of gain- and loss-of-function approaches in chicken and mouse embryonic models, as well as mouse embryonic stem cells (mESCs), we have observed that manipulating the level of netrin1 specifically alters the patterning of the Bmp-dependent dorsal interneurons (dIs), dI1-dI3. Altered netrin1 levels also change Bmp signaling activity, as assessed using bioinformatic approaches, as well as monitoring phosophoSmad1/5/8 activation, the canonical intermediate of Bmp signaling, and Id levels, a known Bmp target. Together, these studies support the hypothesis that netrin1 acts from the intermediate spinal cord to regionally confine Bmp signaling to the dorsal spinal cord. Thus, netrin1 has reiterative activities shaping dorsal spinal circuits, first by regulating cell fate decisions and then acting as a guidance cue to direct axon extension.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
-
Neuroscience
Nesprin-2 coordinates opposing microtubule motors during nuclear migration in neurons.
In The Journal of Cell Biology on 4 November 2024 by Zhou, C., Wu, Y. K., et al.
Nuclear migration is critical for the proper positioning of neurons in the developing brain. It is known that bidirectional microtubule motors are required for nuclear transport, yet the mechanism of the coordination of opposing motors is still under debate. Using mouse cerebellar granule cells, we demonstrate that Nesprin-2 serves as a nucleus-motor adaptor, coordinating the interplay of kinesin-1 and dynein. Nesprin-2 recruits dynein-dynactin-BicD2 independently of the nearby kinesin-binding LEWD motif. Both motor binding sites are required to rescue nuclear migration defects caused by the loss of function of Nesprin-2. In an intracellular cargo transport assay, the Nesprin-2 fragment encompassing the motor binding sites generates persistent movements toward both microtubule minus and plus ends. Nesprin-2 drives bidirectional cargo movements over a prolonged period along perinuclear microtubules, which advance during the migration of neurons. We propose that Nesprin-2 keeps the nucleus mobile by coordinating opposing motors, enabling continuous nuclear transport along advancing microtubules in migrating cells.
© 2024 Zhou et al.
-
IHC
-
Cell Biology
-
Neuroscience
Permanent deconstruction of intracellular primary cilia in differentiating granule cell neurons.
In The Journal of Cell Biology on 7 October 2024 by Ott, C. M., Constable, S., et al.
Primary cilia on granule cell neuron progenitors in the developing cerebellum detect sonic hedgehog to facilitate proliferation. Following differentiation, cerebellar granule cells become the most abundant neuronal cell type in the brain. While granule cell cilia are essential during early developmental stages, they become infrequent upon maturation. Here, we provide nanoscopic resolution of cilia in situ using large-scale electron microscopy volumes and immunostaining of mouse cerebella. In many granule cells, we found intracellular cilia, concealed from the external environment. Cilia were disassembled in differentiating granule cell neurons-in a process we call cilia deconstruction-distinct from premitotic cilia resorption in proliferating progenitors. In differentiating granule cells, cilia deconstruction involved unique disassembly intermediates, and, as maturation progressed, mother centriolar docking at the plasma membrane. Unlike ciliated neurons in other brain regions, our results show the deconstruction of concealed cilia in differentiating granule cells, which might prevent mitogenic hedgehog responsiveness. Ciliary deconstruction could be paradigmatic of cilia removal during differentiation in other tissues.
© 2024 Ott et al.
-
IHC-IF
-
Cell Biology
-
Neuroscience
In NPJ Precis Oncol on 24 September 2022 by Al-Qasem, A. J., Alves, C. L., et al.
Fig.2.F

-
WB
-
Homo sapiens (Human)
Collected and cropped from NPJ Precis Oncol by CiteAb, provided under a CC-BY license
Image 1 of 53
In Cancers (Basel) on 16 August 2022 by Mejía-Hernández, J. O., Raghu, D., et al.
Fig.5.E

-
WB
-
Collected and cropped from Cancers (Basel) by CiteAb, provided under a CC-BY license
Image 1 of 53
In Antioxidants (Basel) on 11 May 2022 by Lastra, D., Escoll, M., et al.
Fig.7.C

-
WB
-
Collected and cropped from Antioxidants (Basel) by CiteAb, provided under a CC-BY license
Image 1 of 53
In Antioxidants (Basel) on 11 May 2022 by Lastra, D., Escoll, M., et al.
Fig.6.D

-
WB
-
Collected and cropped from Antioxidants (Basel) by CiteAb, provided under a CC-BY license
Image 1 of 53
In Int J Biol Sci on 20 April 2021 by Jiang, X., Zhi, X., et al.
Fig.4.A

-
WB
-
Collected and cropped from Int J Biol Sci by CiteAb, provided under a CC-BY license
Image 1 of 53
In Int J Biol Sci on 20 April 2021 by Jiang, X., Zhi, X., et al.
Fig.3.A

-
WB
-
Collected and cropped from Int J Biol Sci by CiteAb, provided under a CC-BY license
Image 1 of 53
In Cell Death Dis on 9 December 2019 by Hong, S., Yi, J. H., et al.
Fig.1.K

-
WB
-
Mus musculus (House mouse)
Collected and cropped from Cell Death Dis by CiteAb, provided under a CC-BY license
Image 1 of 53
In Theranostics on 23 November 2019 by Lian, Y. F., Huang, Y. L., et al.
Fig.5.A

-
WB
-
Collected and cropped from Theranostics by CiteAb, provided under a CC-BY license
Image 1 of 53
In Molecules on 27 August 2019 by Nishikawa, S., Itoh, Y., et al.
Fig.4.D

-
WB
-
Collected and cropped from Molecules by CiteAb, provided under a CC-BY license
Image 1 of 53
In Cancers (Basel) on 13 June 2019 by Lee, Y. T., Lim, S. H., et al.
Fig.2.B

-
WB
-
Mus musculus (House mouse)
Collected and cropped from Cancers (Basel) by CiteAb, provided under a CC-BY license
Image 1 of 53
In Front Cell Dev Biol on 14 May 2019 by Kawauchi, T. & Nabeshima, Y. I.
Fig.2.C

-
WB
-
Mus musculus (House mouse)
Collected and cropped from Front Cell Dev Biol by CiteAb, provided under a CC-BY license
Image 1 of 53
In Elife on 16 April 2019 by Lawton, A. K., Engstrom, T., et al.
Fig.3.B

-
IHC
-
Mus musculus (House mouse)
Collected and cropped from Elife by CiteAb, provided under a CC-BY license
Image 1 of 53
In Biol Open on 16 July 2018 by Ng, M. Y., Gan, Y. H., et al.
Fig.1.D

-
WB
-
Homo sapiens (Human)
Collected and cropped from Biol Open by CiteAb, provided under a CC-BY license
Image 1 of 53
In Biol Open on 16 July 2018 by Ng, M. Y., Gan, Y. H., et al.
Fig.1.B

-
WB
-
Homo sapiens (Human)
Collected and cropped from Biol Open by CiteAb, provided under a CC-BY license
Image 1 of 53
In Biol Open on 16 July 2018 by Ng, M. Y., Gan, Y. H., et al.
Fig.1.A,B,C,D,E

-
WB
-
Homo sapiens (Human)
Collected and cropped from Biol Open by CiteAb, provided under a CC-BY license
Image 1 of 53
In Biol Open on 16 July 2018 by Ng, M. Y., Gan, Y. H., et al.
Fig.1.A

-
WB
-
Homo sapiens (Human)
Collected and cropped from Biol Open by CiteAb, provided under a CC-BY license
Image 1 of 53
In Int J Mol Sci on 6 February 2018 by Mikamo, M., Kitagawa, K., et al.
Fig.4.E

-
IHC
-
Mus musculus (House mouse)
Collected and cropped from Int J Mol Sci by CiteAb, provided under a CC-BY license
Image 1 of 53
In Biochim Biophys Acta Mol Cell Res on 1 January 2018 by Grey, W., Ivey, A., et al.
Fig.5.A

-
WB
-
Mus musculus (House mouse)
Collected and cropped from Biochim Biophys Acta Mol Cell Res by CiteAb, provided under a CC-BY license
Image 1 of 53
In Biochim Biophys Acta Mol Cell Res on 1 January 2018 by Grey, W., Ivey, A., et al.
Fig.1.C

-
WB
-
Mus musculus (House mouse)
Collected and cropped from Biochim Biophys Acta Mol Cell Res by CiteAb, provided under a CC-BY license
Image 1 of 53
In PLoS One on 1 September 2017 by Pavlidou, T., Rosina, M., et al.
Fig.5.B

-
WB
-
Mus musculus (House mouse)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 53
In PLoS One on 1 September 2017 by Pavlidou, T., Rosina, M., et al.
Fig.4.A

-
WB
-
Mus musculus (House mouse)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 53
In J Biol Chem on 12 May 2017 by Juhasz, A., Markel, S., et al.
Fig.2.C

-
WB
-
Homo sapiens (Human)
Collected and cropped from J Biol Chem by CiteAb, provided under a CC-BY license
Image 1 of 53
In Breast Cancer Res on 16 November 2016 by Griffin, N. I., Sharma, G., et al.
Fig.2.F

-
WB
-
Homo sapiens (Human)
Collected and cropped from Breast Cancer Res by CiteAb, provided under a CC-BY license
Image 1 of 53
In Breast Cancer Res on 16 November 2016 by Griffin, N. I., Sharma, G., et al.
Fig.2.D

-
WB
-
Homo sapiens (Human)
Collected and cropped from Breast Cancer Res by CiteAb, provided under a CC-BY license
Image 1 of 53
In Oncotarget on 20 October 2015 by Jiang, Q. W., Cheng, K. J., et al.
Fig.6.C

-
WB
-
Homo sapiens (Human)
Collected and cropped from Oncotarget by CiteAb, provided under a CC-BY license
Image 1 of 53