Mammalian receptor-mediated endocytosis (RME) often involves at least one of three isoforms of the large GTPase dynamin (Dyn). Dyn pinches-off vesicles at the plasma membrane and mediates uptake of many viruses, although some viruses directly penetrate the plasma membrane. RME is classically interrogated by genetic and pharmacological interference, but this has been hampered by undesired effects. Here we studied virus entry in conditional genetic knock-out (KO) mouse embryonic fibroblasts lacking expression of all three dynamin isoforms (Dyn-KO-MEFs). The small canine parvovirus known to use a single receptor, transferrin receptor, strictly depended on dynamin. Larger viruses or viruses known to use multiple receptors, including alphaviruses, influenza, vesicular stomatitis, bunya, adeno, vaccinia, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and rhinoviruses infected Dyn-KO-MEFs, albeit at higher dosage than wild-type MEFs. In absence of the transmembrane protease serine subtype 2 (TMPRSS2), which normally activates the SARS-CoV-2 spike protein for plasma membrane fusion, SARS-CoV-2 infected angiotensin-converting enzyme 2 (ACE2)-expressing MEFs predominantly through dynamin- and actin-dependent endocytosis. In presence of TMPRSS2 the ancestral Wuhan-strain bypassed both dynamin-dependent and -independent endocytosis, and was less sensitive to endosome maturation inhibitors than the Omicron B1 and XBB variants, supporting the notion that the Omicron variants do not efficiently use TMPRSS2. Collectively, our study suggests that dynamin function at endocytic pits can be essential for infection with single-receptor viruses, while it is not essential but increases uptake and infection efficiency of multi-receptor viruses that otherwise rely on a functional actin network for infection.
Copyright: © 2024 Ojha et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Product Citations: 38
Dynamin independent endocytosis is an alternative cell entry mechanism for multiple animal viruses.
In PLoS Pathogens on 1 November 2024 by Ojha, R., Jiang, A., et al.
-
WB
-
Mus musculus (House mouse)
-
Immunology and Microbiology
In International Journal of Molecular Sciences on 1 August 2024 by Hendricks, E. L., Linskey, N., et al.
The transmembrane protein β-amyloid precursor protein (APP) is central to the pathophysiology of Alzheimer's disease (AD). The β-amyloid hypothesis posits that aberrant processing of APP forms neurotoxic β-amyloid aggregates, which lead to the cognitive impairments observed in AD. Although numerous additional factors contribute to AD, there is a need to better understand the synaptic function of APP. We have found that Drosophila APP-like (APPL) has both shared and non-shared roles at the synapse with Kismet (Kis), a chromatin helicase binding domain (CHD) protein. Kis is the homolog of CHD7 and CHD8, both of which are implicated in neurodevelopmental disorders including CHARGE Syndrome and autism spectrum disorders, respectively. Loss of function mutations in kis and animals expressing human APP and BACE in their central nervous system show reductions in the glutamate receptor subunit, GluRIIC, the GTPase Rab11, and the bone morphogenetic protein (BMP), pMad, at the Drosophila larval neuromuscular junction (NMJ). Similarly, processes like endocytosis, larval locomotion, and neurotransmission are deficient in these animals. Our pharmacological and epistasis experiments indicate that there is a functional relationship between Kis and APPL, but Kis does not regulate appl expression at the larval NMJ. Instead, Kis likely influences the synaptic localization of APPL, possibly by promoting rab11 transcription. These data identify a potential mechanistic connection between chromatin remodeling proteins and aberrant synaptic function in AD.
-
ICC
Alternative cell entry mechanisms for SARS-CoV-2 and multiple animal viruses
Preprint on BioRxiv : the Preprint Server for Biology on 3 July 2023 by Ojha, R., Jiang, A., et al.
The cell entry mechanism of SARS-CoV-2, the causative agent of the COVID-19 pandemic, is not fully understood. Most animal viruses hijack cellular endocytic pathways as an entry route into the cell. Here, we show that in cells that do not express serine proteases such as TMPRSS2, genetic depletion of all dynamin isoforms blocked the uptake and strongly reduced infection with SARS-CoV-2 and its variant Delta. However, increasing the viral loads partially and dose-dependently restored infection via a thus far uncharacterized entry mechanism. Ultrastructural analysis by electron microscopy showed that this dynamin-independent endocytic processes appeared as 150-200 nm non-coated invaginations and was efficiently used by numerous mammalian viruses, including alphaviruses, influenza, vesicular stomatitis, bunya, adeno, vaccinia, and rhinovirus. Both the dynamin-dependent and dynamin-independent infection of SARS-CoV-2 required a functional actin cytoskeleton. In contrast, the alphavirus Semliki Forest virus, which is smaller in diameter, required actin only for the dynamin-independent entry. The presence of TMPRSS2 protease rescued SARS-CoV-2 infection in the absence of dynamins. Collectively, these results indicate that some viruses such as canine parvovirus and SARS-CoV-2 mainly rely on dynamin for endocytosis-dependent infection, while other viruses can efficiently bypass this requirement harnessing an alternative infection entry route dependent on actin.
-
COVID-19
-
Immunology and Microbiology
Trafficking proteins show limited differences in mobility across different postsynaptic spines.
In IScience on 17 February 2023 by Mougios, N., Opazo, F., et al.
The function of the postsynaptic compartment is based on the presence and activity of postsynaptic receptors, whose dynamics are controlled by numerous scaffolding, signaling and trafficking proteins. Although the receptors and the scaffolding proteins have received substantial attention, the trafficking proteins have not been investigated extensively. Their mobility rates are unknown, and it is unclear how the postsynaptic environment affects their dynamics. To address this, we analyzed several trafficking proteins (α-synuclein, amphiphysin, calmodulin, doc2a, dynamin, and endophilin), estimating their movement rates in the dendritic shaft, as well as in morphologically distinct "mushroom" and "stubby" postsynapse types. The diffusion parameters were surprisingly similar across dendritic compartments, and a few differences between proteins became evident only in the presence of a synapse neck. We conclude that the movement of trafficking proteins is not strongly affected by the postsynaptic compartment, in stark contrast to the presynapse, which regulates strongly the movement of such proteins.
© 2023 The Author(s).
-
Rattus norvegicus (Rat)
In Nature Communications on 24 November 2022 by Matthaeus, C., Sochacki, K. A., et al.
Caveolae are small coated plasma membrane invaginations with diverse functions. Caveolae undergo curvature changes. Yet, it is unclear which proteins regulate this process. To address this gap, we develop a correlative stimulated emission depletion (STED) fluorescence and platinum replica electron microscopy imaging (CLEM) method to image proteins at single caveolae. Caveolins and cavins are found at all caveolae, independent of curvature. EHD2 is detected at both low and highly curved caveolae. Pacsin2 associates with low curved caveolae and EHBP1 with mostly highly curved caveolae. Dynamin is absent from caveolae. Cells lacking dynamin show no substantial changes to caveolae, suggesting that dynamin is not directly involved in caveolae curvature. We propose a model where caveolins, cavins, and EHD2 assemble as a cohesive structural unit regulated by intermittent associations with pacsin2 and EHBP1. These coats can flatten and curve to enable lipid traffic, signaling, and changes to the surface area of the cell.
© 2022. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
-
WB
In Front Cell Neurosci on 16 March 2021 by Huang, G. & Eckrich, S.
Fig.7.C

-
IHC-IF
-
Mus musculus (House mouse)
Collected and cropped from Front Cell Neurosci by CiteAb, provided under a CC-BY license
Image 1 of 2
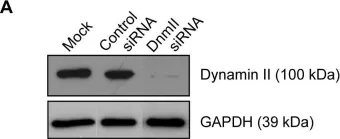
In PLoS One on 25 December 2013 by Lum, M., Attridge, S. R., et al.
Fig.2.A

-
WB
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 2