Human papillomavirus (HPV) type 16 is the most common sexually transmitted virus related to cervical cancer. Among different types of advanced novel therapies, the clustered regularly interspaced short palindromic repeat (CRISPR)/Cas-mediated gene editing holds great promise for cancer treatment. In this research, optimal gRNA sequences targeting HPV16 E5, E6, E7, and p97 promoter for CRISPR/Cas9-mediated genome editing were designed by in silico prediction. After cloning, delivery of the recombinant vectors into C3, TC1 and HeLa tumor cells was evaluated by Lipofectamine 2000, and LL-37 antimicrobial peptide. Then, the levels of cell cycle proteins (p21, p53, and Rb) were investigated after treatment by western blot analysis. Finally, C57BL/6 mice were inoculated with C3 tumor cells, and treated with recombinant vectors and cisplatin. Based on the tumor size reduction and IHC results, the E6 + E7-treated group with a high percentage of cleaved caspase-3 positive cells (45.75%) and low mitotic index of 2-3 was determined as the best treatment among other groups. Moreover, the potential of LL-37 peptide to overcome the CRISPR/Cas9 delivery challenge was shown for the first time. Overall, our study suggests that the CRISPR/Cas9-mediated gene editing of pre-existing tumors is effective, specific and nontoxic, and the outlook for precise gene therapy in cancer patients is very bright.
© 2023 Wiley Periodicals LLC.
Product Citations: 27
In Journal of Medical Virology on 1 July 2023 by Khairkhah, N., Bolhassani, A., et al.
-
WB
-
Homo sapiens (Human)
-
Immunology and Microbiology
In PLoS ONE on 23 November 2022 by Nakanoh, S., Kadiwala, J., et al.
RB is a well-known cell cycle regulator controlling the G1 checkpoint. Previous reports have suggested that it can influence cell fate decisions not only by regulating cell proliferation and survival but also by interacting with transcription factors and epigenetic modifiers. However, the functional redundancy of RB family proteins (RB, RBL1 and RBL2) renders it difficult to investigate their roles during early development, especially in human. Here, we address this problem by generating human embryonic stem cells lacking RB family proteins. To achieve this goal, we first introduced frameshift mutations in RBL1 and RBL2 genes using the CRISPR/Cas9 technology, and then integrated the shRNA-expression cassette to knockdown RB upon tetracycline treatment. The resulting RBL1/2_dKO+RB_iKD cells remain pluripotent and efficiently differentiate into the primary germ layers in vitro even in the absence of the RB family proteins. In contrast, we observed that subsequent differentiation into foregut endoderm was impaired without the expression of RB, RBL1 and RBL2. Thus, it is suggested that RB proteins are dispensable for the maintenance and acquisition of cell identities during early development, but they are essential to generate advanced derivatives after the formation of primary germ layers. These results also indicate that our RBL1/2_dKO+RB_iKD cell lines are useful to depict the detailed molecular roles of RB family proteins in the maintenance and generation of various cell types accessible from human pluripotent stem cells.
Copyright: © 2022 Nakanoh et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
-
WB
-
Homo sapiens (Human)
-
Stem Cells and Developmental Biology
In PLoS ONE on 8 January 2021 by Jubair, L., Lam, A. K., et al.
Gene-editing has raised the possibility of being able to treat or cure cancers, but key challenges remain, including efficient delivery, in vivo efficacy, and its safety profile. Ideal targets for cancer therapy are oncogenes, that when edited, cause cell death. Here, we show, using the human papillomavirus (HPV) type 16 cancer cell line TC1, that CRISPR/Cas9 targeting the E7 oncogene and packaged in PEGylated liposomes cleared established tumours in immunocompetent mice. Treatment caused no significant toxicity in the spleen or liver. An ideal therapeutic outcome would be the induction of an immunogenic cell death (ICD), such that recurrent tumours would be eliminated by the host immune system. We show here for the first time that CRISPR/Cas9-mediated cell death via targeting E7 did not result in ICD. Overall, our data show that in vivo CRISPR/Cas targeting of oncogenes is an effective treatment approach for cancer.
-
WB
-
Cancer Research
In Molecular Therapy on 4 December 2019 by Jubair, L., Fallaha, S., et al.
The recent advancements in CRISPR/Cas9 engineering have resulted in the development of more targeted and potentially safer gene therapies. The challenge in the cancer setting is knowing the driver oncogenes responsible, and the translation of these therapies is hindered by effective and safe delivery methods to target organs with minimal systemic toxicities, on-target specificity of gene editing, and demonstrated lack of long-term adverse events. Using a model system based on cervical cancer, which is driven by the ongoing expression of the human papillomavirus E6 and E7 proteins, we show that CRISPR/Cas9 delivered systemically in vivo using PEGylated liposomes results in tumor elimination and complete survival in treated animals. We compared treatment and editing efficiency of two Cas9 variants, wild-type (WT) Cas9 and the highly specific FokI-dCas9, and showed that the latter was not effective. We also explored high-fidelity repair but found that repair was inefficient, occurring in 6%-8% of cells, whereas non-homologous end joining (NHEJ) was highly efficient, occurring in ∼80% of the cells. Finally, we explored the post gene-editing events in tumors and showed that cell death is induced by apoptosis. Overall, our work demonstrates that in vivo CRISPR/Cas editing treatment of preexisting tumors is completely effective despite the large payloads.
Crown Copyright © 2019. Published by Elsevier Inc. All rights reserved.
-
WB
-
Homo sapiens (Human)
-
Cancer Research
In Cell Cycle on 1 January 2019 by Vasjari, L., Bresan, S., et al.
Numerous studies exploring oncogenic Ras or manipulating physiological Ras signalling have established an irrefutable role for Ras as driver of cell cycle progression. Despite this wealth of information the precise signalling timeline and effectors engaged by Ras, particularly during G1, remain obscure as approaches for Ras inhibition are slow-acting and ill-suited for charting discrete Ras signalling episodes along the cell cycle. We have developed an approach based on the inducible recruitment of a Ras-GAP that enforces endogenous Ras inhibition within minutes. Applying this strategy to inhibit Ras stepwise in synchronous cell populations revealed that Ras signaling was required well into G1 for Cyclin D induction, pocket protein phosphorylation and S-phase entry, irrespective of whether cells emerged from quiescence or G2/M. Unexpectedly, Erk, and not PI3K/Akt or Ral was activated by Ras at mid-G1, albeit PI3K/Akt signalling was a necessary companion of Ras/Erk for sustaining cyclin-D levels and G1/S transition. Our findings chart mitogenic signaling by endogenous Ras during G1 and identify limited effector engagement restricted to Raf/MEK/Erk as a cogent distinction from oncogenic Ras signalling.
-
WB
-
Cell Biology
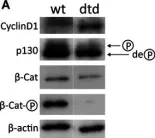
In J Cell Biochem on 1 October 2014 by De Leonardis, F., Monti, L., et al.
Fig.1.A

-
WB
-
Mus musculus (House mouse)
Collected and cropped from J Cell Biochem by CiteAb, provided under a CC-BY license
Image 1 of 3
In PLoS Genet on 1 September 2014 by Iannetti, A., Ledoux, A. C., et al.
Fig.7.A

-
WB
-
Collected and cropped from PLoS Genet by CiteAb, provided under a CC-BY license
Image 1 of 3
In BMC Cancer on 25 August 2011 by Wedel, S., Hudak, L., et al.
Fig.2.A

-
WB
-
Homo sapiens (Human)
Collected and cropped from BMC Cancer by CiteAb, provided under a CC-BY license
Image 1 of 3