Endothelial cells (EC) play a pivotal role in vascular homeostasis. By sensing shear stress generated by blood flow, EC endorse vasculoprotection through mechanotransduction signaling pathways. Various ion channels are involved in mechanosignaling, and here, we investigated the endothelial voltage-gated Na+ channels (NaV channels), since their mechanosensitivity has been previously demonstrated in cardiomyocytes. First, we showed that EC from aorta (TeloHAEC) behave as EC from umbilical vein (HUVEC) under laminar shear stress (LSS). For both EC models, cell alignment and elongation occurred with the activation of the KLF2/KLF4 atheroprotective signaling pathways. We found that LSS decreased the expression of SCN5A, encoding NaV1.5, while LSS increased that of SCN3B, encoding NaVβ3. We demonstrated that the KLF4 transcription factor is involved in SCN3B expression under both static and LSS conditions. Interestingly, SCN3B silencing impaired EC alignment induced by LSS. The characterization of NaVβ3 interactome by coimmunoprecipitation and proteomic analysis revealed that mTOR, implicated in autophagy, binds to NaVβ3. This result was evidenced by the colocalization between NaVβ3 and mTOR inside cells. Moreover, we showed that SCN3B silencing led to the decrease in LC3B expression and the number of LC3B positive autophagosomes. Furthermore, we showed that NaVβ3 is retained within the cell and colocalized with LAMP1 and LC3B. Finally, we found that resveratrol, a stimulating-autophagy and vasculoprotective molecule, induced KLF4 together with NaVβ3 expression. Altogether, our findings highlight a novel role of NaVβ3 in endothelial function and cell alignment as an actor in shear stress vasculoprotective intracellular pathway through autophagy modulation.
© 2025 The Author(s). The FASEB Journal published by Wiley Periodicals LLC on behalf of Federation of American Societies for Experimental Biology.
Product Citations: 351
In The FASEB Journal on 15 June 2025 by Réthoré, L., Guihot, A. L., et al.
-
Cell Biology
In Stem Cell Research & Therapy on 2 June 2025 by O'Neill, K. M., Edgar, K. S., et al.
Progenitor endothelial colony forming cells (ECFCs) are critical for vascular homeostasis and hold therapeutic potential for ischaemic cardiovascular disease (CVD). As angiogenic capacity and efficacy within diseased tissues is particularly impacted in diabetic patients, who show high incidence of ischaemic CVD, targeting of critical ECFC pathways in this setting represents an innovative focus towards enhancing intrinsic vasoreparative function. We previously reported that NADPH oxidase 4 (NOX4)-derived reactive oxygen species promote cord blood-derived ECFC (CB-ECFC) pro-angiogenic response, whilst NOX4 overexpression (OE) enhances revascularisation capacity. Here, we aimed to investigate specific influence of NOX4-dependent signalling on CB-ECFC angiogenic dysfunction observed upon exposure to both experimental and clinical diabetes to define whether NOX4 may represent a viable therapeutic target in this context.
CB-ECFCs were cultured in high glucose (D-glucose, 25 mmol/L) or control media (5 mmol/L) ± phorbol 12-myristate 13- acetate (PMA, 500 nmol/L) for 72 h with assessment of migratory/tubulogenic capacity and NOX4 mRNA expression (qRT-PCR). Detailed analysis of angiogenic function and signalling (Western blot, RNA sequencing) was performed in CB-ECFCs isolated from donors with gestational diabetes prior to NOX4 plasmid OE to define rescue potential and key mechanistic pathways (network analysis, proteome profiling). Statistical significance was determined using one-way ANOVA with Bonferroni post-host testing or paired/unpaired Student's t-test, as appropriate.
PMA-stimulated CB-ECFC migration and tube-forming capacity observed in control cells was suppressed in experimental diabetes in parallel with reduced NOX4 expression and rescued by plasmid NOX4OE. As direct evidence of clinical relevance, CB-ECFCs from gestational diabetic donors showed reduced angiogenic potential associated with attenuated NOX4, eNOS activity and downregulation of key vasoreparative signalling. Furthermore, NOX4OE rescued angiogenic function in chronically diabetic CB-ECFCs via modulation of downstream signalling involving both direct and indirect enhancement of pro-angiogenic protein expression (endoglin/SERPINE1/E2F1) linked to reduced p53 phosphorylation.
Taken together, these data indicate for the first time that reduced NOX4 expression plays a pivotal role in CB-ECFC angiogenic dysfunction linked with diabetes whilst highlighting NOX4-dependent signalling as a potential target to protect and augment their intrinsic vasoreparative capacity towards addressing current translational barriers.
© 2025. The Author(s).
-
Stem Cells and Developmental Biology
In Redox Biology on 1 May 2025 by Zhang, Y., Youn, J. Y., et al.
We and others have previously shown that uncoupling of endothelial nitric oxide synthase (eNOS) induces oxidative stress in diabetes, contributing to endothelial dysfunction. Activation of NADPH oxidase (NOX) isoform NOX1 by angiotensin II (Ang II) triggers eNOS uncoupling via deficiency in dihydrofolate reductase (DHFR) in streptozotocin (STZ)-induced type 1 diabetic mice. Presently, we investigated whether accelerated atherosclerosis is attenuated in apoE/NOX1 double knockout, and whether mice overexpressing DHFR in the endothelium (tg-EC-DHFR, generated in house) recouples eNOS to alleviate diabetic atherogenesis. At baseline, endothelial-specific DHFR overexpression recoupled eNOS and improved vasorelaxation in the aortas and mesenteric arteries of STZ-induced diabetic mice. Accelerated atherogenesis in STZ/high-fat diet (HFD) treated apoE-/- mice was markedly abrogated in tg-EC-DHFR background, establishing an important role of endothelial DHFR in maintaining vascular function and protecting from diabetic atherogenesis. Moreover, by crossing apoE-/- with NOX1 null mice (NOX1-/y), we found that NOX1 deletion markedly diminished atherosclerotic lesion formation in HFD + STZ-treated apoE-/-/NOX1-/y mice, indicating that NOX1 lies upstream of eNOS uncoupling in facilitating diabetic atherogenesis. Oral administration with folic acid (FA), shown to upregulate DHFR, robustly attenuated atherosclerotic lesion formation in HFD + STZ-treated apoE-/- mice similarly to NOX1 deletion. Taken together, our data for the first time demonstrate that endothelial DHFR plays an important role in the preservation of endothelial function and inhibition of atherosclerosis in diabetes, deficiency of which consequent to NOX1 activation mediates eNOS uncoupling driven lesion formation. Strategies targeting uncoupled eNOS prove to be robust treatment options for diabetic endothelial dysfunction and atherogenesis.
Copyright © 2025 The Authors. Published by Elsevier B.V. All rights reserved.
In Physiological Reports on 1 April 2025 by Ray, J. W., Sun, X., et al.
The mechanisms of sex differences in cerebrovascular function are not well understood. In this study, we determined whether sex differences in middle cerebral artery (MCA) reactivity are accompanied with changes in cerebral or systemic arterial resistance and stiffness in young adult Sprague-Dawley (SD) rats. No differences in systolic or diastolic blood pressures were observed between sexes. Heart rate was higher in the female versus male SD. Left MCA pulsatility index (PI) was lower in female versus male SD. No differences in left intracranial internal carotid artery (ICA) PI were observed between sexes. There were no differences in thoracic aorta or left common carotid artery pulse wave velocity (PWV) between sexes. In isolated MCA segments, female left MCA had lower contraction to potassium, but similar maximal contraction and sensitivity to thromboxane A2 receptor agonist U46619. Pre-incubation with indomethacin lowered maximal response and sensitivity to U46619 in male but not female MCA. Endothelial nitric oxide synthase and vascular smooth muscle layer thromboxane A2 receptor immunoreactivity were greater in female versus male SD. We conclude that sex differences in the MCA reactivity are associated with a differential functional profile of MCA in adult SD rats independent from changes in systemic PWV.
© 2025 The Author(s). Physiological Reports published by Wiley Periodicals LLC on behalf of The Physiological Society and the American Physiological Society.
-
Cardiovascular biology
Pulsatile-flow culture: a novel system for assessing vascular-cell dynamics.
In Lab On A Chip on 25 March 2025 by Salimi-Afjani, N., Rieben, R., et al.
We describe a model system for vascular-cell culture where recirculating fluid flow in standard culture plates is generated by gravity using a combination of platform tilt and rotation (nutation). Placed inside a cell-culture incubator, variable nutation speeds provide pulsatile shear stresses to vascular cells within the physiological range. The effect of these stresses on cells is demonstrated here using standard laboratory techniques such as immunofluorescent staining, immunoblot, and supernatant analyses. This gravity-driven model framework is well-suited for assessing dynamic conditions for mono- and co-cultures. In addition, the modular design and the use of off-the-shelf components make the system economical and scalable.
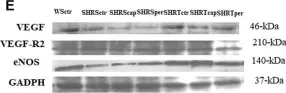
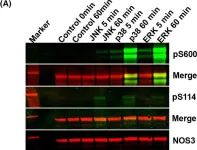
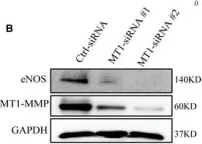
In Int J Mol Sci on 15 March 2024 by Wong, N. K. P., Solly, E. L., et al.
Fig.5.I

-
WB
-
Collected and cropped from Int J Mol Sci by CiteAb, provided under a CC-BY license
Image 1 of 39
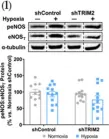
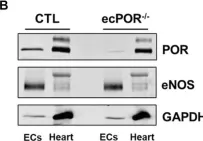
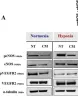
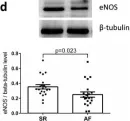
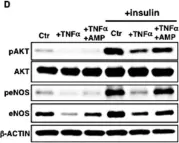
In Front Physiol on 7 June 2023 by Macedo, A. G., Miotto, D. S., et al.
Fig.2.D

-
WB
-
Collected and cropped from Front Physiol by CiteAb, provided under a CC-BY license
Image 1 of 39
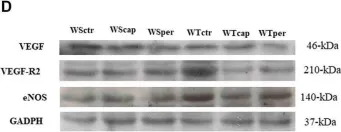
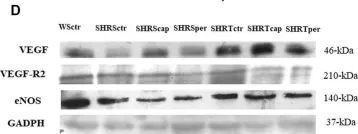
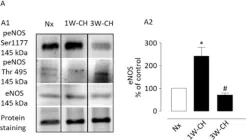
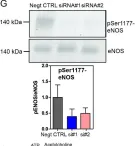
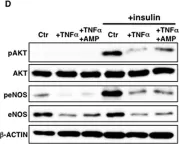
In Front Physiol on 7 June 2023 by Macedo, A. G., Miotto, D. S., et al.
Fig.4.D

-
WB
-
Collected and cropped from Front Physiol by CiteAb, provided under a CC-BY license
Image 1 of 39
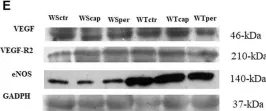
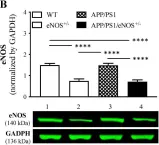
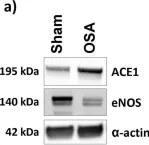
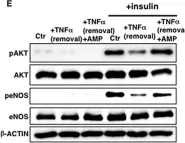
In Front Physiol on 7 June 2023 by Macedo, A. G., Miotto, D. S., et al.
Fig.5.E

-
WB
-
Collected and cropped from Front Physiol by CiteAb, provided under a CC-BY license
Image 1 of 39
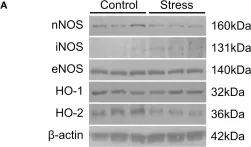
In Front Physiol on 7 June 2023 by Macedo, A. G., Miotto, D. S., et al.
Fig.6.E

-
WB
-
Collected and cropped from Front Physiol by CiteAb, provided under a CC-BY license
Image 1 of 39
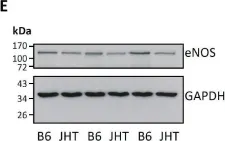
In Front Physiol on 20 December 2022 by Lopez, M., Malacarne, P. F., et al.
Fig.1.B

-
WB
-
Collected and cropped from Front Physiol by CiteAb, provided under a CC-BY license
Image 1 of 39
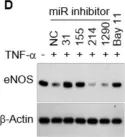
In Cells on 30 July 2022 by Porto Ribeiro, T., Barbeau, S., et al.
Fig.8.A

-
WB
-
Collected and cropped from Cells by CiteAb, provided under a CC-BY license
Image 1 of 39
In Int J Mol Sci on 30 June 2022 by Ahmed, S., Jing, Y., et al.
Fig.1.B

-
WB
-
Mus musculus (House mouse)
Collected and cropped from Int J Mol Sci by CiteAb, provided under a CC-BY license
Image 1 of 39
In FEBS Open Bio on 1 May 2022 by Solone, X. K. V., Caldara, A. L., et al.
Fig.3.A

-
WB
-
Collected and cropped from FEBS Open Bio by CiteAb, provided under a CC-BY license
Image 1 of 39
In Int J Mol Sci on 20 March 2022 by Mulangala, J., Akers, E. J., et al.
Fig.5.A

-
WB
-
Mus musculus (House mouse)
Collected and cropped from Int J Mol Sci by CiteAb, provided under a CC-BY license
Image 1 of 39
In Biomedicines on 14 November 2021 by Xia, N., Hasselwander, S., et al.
Fig.1.E

-
WB
-
Collected and cropped from Biomedicines by CiteAb, provided under a CC-BY license
Image 1 of 39
In Cells on 31 August 2020 by Kim, S., Park, M., et al.
Fig.5.D

-
WB
-
Collected and cropped from Cells by CiteAb, provided under a CC-BY license
Image 1 of 39
In EMBO Mol Med on 7 February 2020 by Esteban, S., Clemente, C., et al.
Fig.4.B

-
WB
-
Homo sapiens (Human)
Collected and cropped from EMBO Mol Med by CiteAb, provided under a CC-BY license
Image 1 of 39
In J Clin Med on 23 December 2019 by Jesel, L., Abbas, M., et al.
Fig.1.D

-
WB
-
Homo sapiens (Human)
Collected and cropped from J Clin Med by CiteAb, provided under a CC-BY license
Image 1 of 39
In PLoS One on 11 October 2019 by Matthaeus, C., Lian, X., et al.
Fig.5.G

-
WB
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 39
In Sci Rep on 7 August 2019 by Rubies, C., Dantas, A. P., et al.
Fig.5.A

-
WB
-
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 39
In JACC Basic Transl Sci on 1 August 2019 by Lotteau, S., Ivarsson, N., et al.
Fig.3.B

-
WB
-
Collected and cropped from JACC Basic Transl Sci by CiteAb, provided under a CC-BY license
Image 1 of 39
In J Clin Invest on 1 February 2019 by Osmanagic-Myers, S., Kiss, A., et al.
Fig.4.B

-
WB
-
Collected and cropped from J Clin Invest by CiteAb, provided under a CC-BY license
Image 1 of 39
In PLoS One on 8 February 2018 by Tsuboi, T., Maeda, M., et al.
Fig.4.A

-
WB
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 39
In Front Physiol on 30 January 2018 by Pedard, M., Quirié, A., et al.
Fig.4.A

-
WB
-
Rattus norvegicus (Rat)
Collected and cropped from Front Physiol by CiteAb, provided under a CC-BY license
Image 1 of 39
In Oncotarget on 19 January 2018 by Sasaki, N., Itakura, Y., et al.
Fig.2.D

-
WB
-
Collected and cropped from Oncotarget by CiteAb, provided under a CC-BY license
Image 1 of 39
In Oncotarget on 19 January 2018 by Sasaki, N., Itakura, Y., et al.
Fig.3.D

-
WB
-
Collected and cropped from Oncotarget by CiteAb, provided under a CC-BY license
Image 1 of 39
In Oncotarget on 19 January 2018 by Sasaki, N., Itakura, Y., et al.
Fig.5.D

-
WB
-
Collected and cropped from Oncotarget by CiteAb, provided under a CC-BY license
Image 1 of 39
In Oncotarget on 19 January 2018 by Sasaki, N., Itakura, Y., et al.
Fig.6.E

-
WB
-
Collected and cropped from Oncotarget by CiteAb, provided under a CC-BY license
Image 1 of 39
In Front Neurosci on 19 January 2016 by Lee, S., Kang, B. M., et al.
Fig.4.A

-
WB
-
Collected and cropped from Front Neurosci by CiteAb, provided under a CC-BY license
Image 1 of 39