This study investigates the impact of Titin (TTN) gene mutations on radiotherapy sensitivity in rectum adenocarcinoma (READ) by examining changes in the tumour immune microenvironment.
Data on gene expression and mutations in READ were obtained from The Cancer Genome Atlas (TCGA) and International Cancer Genome Consortium (ICGC) databases. Bioinformatics analysis explored the correlation between TTN mutations and immune cell infiltration. In vitro, lentiviral vectors were used to assess TTN mutations' effects on ANKRD1 expression in two READ cell lines. ANKRD1 was overexpressed, and clonogenic assays evaluated radiotherapy sensitivity. Flow cytometry, immunofluorescence, and comet assays examined mutations' impact on cell cycle, apoptosis, and DNA damage response (DDR). An in vivo mouse model and formalin-fixed paraffin-embedded samples from locally advanced rectal cancer (LARC) patients before and after radiotherapy were analyzed, followed by prognostic evaluation.
Bioinformatics revealed that TTN mutations increase radiation sensitivity in LARC by slowing cell proliferation, promoting apoptosis, and reducing DDR. TTN mutations also inhibit ANKRD1 expression via JUN disruption and enhance CD4/CD8 T-cell infiltration, improving anti-tumour immunity and outcomes. Observations from the clinical study showed a substantial decline in ANKRD1 expression levels alongside a notable surge in the counts of CD4+ and CD8+ T cells after undergoing radiotherapy. Patients with TTN mutations, low ANKRD1 expression, and high densities of CD4+ and CD8+ T cells had longer 3-year disease-free survival in READ.
Our findings reveal that TTN mutations can serve as biomarkers for enhanced radiotherapy sensitivity in READ. By altering the tumour's immune microenvironment, these mutations may provide a novel target for personalized radiotherapy strategies, potentially improving therapeutic outcomes in patients with READ.
The association between TTN mutations and tumour mutation burden, as well as immune cell infiltration in READ, is examined. TTN mutations enhance the radiation sensitivity of READ cells and weaken DNA damage repair in response to radiation. TTN mutations increase the radiation sensitivity of READ cells by inhibiting ANKRD1. The infiltration of CD8+ and CD4+ T cells induced by TTN mutations is essential for anti-tumour immunity. TTN mutations serve as a biomarker for the pathological response to preoperative radiotherapy in READ.
© 2025 The Author(s). Clinical and Translational Medicine published by John Wiley & Sons Australia, Ltd on behalf of Shanghai Institute of Clinical Bioinformatics.
Product Citations: 51
In Clinical and Translational Medicine on 1 January 2025 by Liu, H., Liu, J., et al.
-
Cancer Research
-
Immunology and Microbiology
In ACS Omega on 2 July 2024 by Stulpinas, A., Tenkutytė, M., et al.
Toxicity and the emergence of resistance are the main challenges in cancer treatment. The optimal dose of cisplatin, one of the most widely used chemotherapeutic anticancer drugs, is currently being widely debated. Furthermore, the dose-dependent molecular mechanisms of its action are poorly understood. To assess the role of protein kinase JNK (cJun N-terminal kinase) signaling in lung cancer treatment, we combined small-molecule JNK inhibitors and cisplatin. Wild-type p53 (tumor suppressor transcription factor TP53) and mutated RAS-bearing lung adenocarcinoma cell line A549 was used as a model in our studies. Here, we demonstrate cisplatin concentration-dependent opposing roles of JNK in killing cancer cells: a cell-protective role at low cisplatin concentrations and an apoptosis-promoting (or neutral) role at high concentrations. Time- and dose-dependent activation of pro-survival protein kinase AKT and TP53 was shown, with similar activation dynamics in cells exposed to different (low and high) cisplatin concentrations. Selective inhibition of AKT and activation of TP53 (expression and phosphorylation) led to a decrease in cell survival, indicating their involvement in cisplatin-induced cell death regulation. The activation levels of TP53 and AKT in cisplatin-treated A549 cells after cotreatment with the JNK inhibitor SP600125 correlated with their role in regulating cell death. TP53 and AKT were proposed as signaling proteins mediating the outcome of JNK inhibition in A549 cells exposed to different concentrations of cisplatin. Our findings suggest that a combination of stress kinase JNK inhibition and low-dose cisplatin, together with manipulation of drug-induced signaling, could be considered as a promising treatment strategy for certain lung cancers.
© 2024 The Authors. Published by American Chemical Society.
-
Homo sapiens (Human)
-
Cancer Research
In The Journal of Steroid Biochemistry and Molecular Biology on 1 June 2024 by Kermpatsou, D., Olsson, F., et al.
The active form of vitamin D, 1,25-dihydroxyvitamin D3, is known to act via VDR (vitamin D receptor), affecting several physiological processes. In addition, PDIA3 (protein disulphide-isomerase A3) has been associated with some of the functions of 1,25-dihydroxyvitamin D3. In the present study we used siRNA-mediated silencing of PDIA3 in osteosarcoma and prostate carcinoma cell lines to examine the role(s) of PDIA3 for 1,25-dihydroxyvitamin D3-dependent responses. PDIA3 silencing affected VDR target genes and significantly altered the 1,25-dihydroxyvitamin D3-dependent induction of CYP24A1, essential for elimination of excess 1,25-dihydroxyvitamin D3. Also, PDIA3 silencing significantly altered migration and proliferation in prostate PC3 cells, independently of 1,25-dihydroxyvitamin D3. 1,25-Dihydroxyvitamin D3 increased thermostability of PDIA3 in cellular thermal shift assay, supporting functional interaction between PDIA3 and 1,25-dihydroxyvitamin D3-dependent pathways. In summary, our data link PDIA3 to 1,25-dihydroxyvitamin D3-mediated signalling, underline and extend its role in proliferation and reveal a novel function in maintenance of 1,25-dihydroxyvitamin D3 levels.Copyright © 2024 The Authors. Published by Elsevier Ltd.. All rights reserved.
-
Biochemistry and Molecular biology
In eLife on 8 March 2024 by Grove, M., Kim, H., et al.
Previously we showed that the hippo pathway transcriptional effectors, YAP and TAZ, are essential for Schwann cells (SCs) to develop, maintain and regenerate myelin . Although TEAD1 has been implicated as a partner transcription factor, the mechanisms by which it mediates YAP/TAZ regulation of SC myelination are unclear. Here, using conditional and inducible knockout mice, we show that TEAD1 is crucial for SCs to develop and regenerate myelin. It promotes myelination by both positively and negatively regulating SC proliferation, enabling Krox20/Egr2 to upregulate myelin proteins, and upregulating the cholesterol biosynthetic enzymes FDPS and IDI1. We also show stage-dependent redundancy of TEAD1 and that non-myelinating SCs have a unique requirement for TEAD1 to enwrap nociceptive axons in Remak bundles. Our findings establish TEAD1 as a major partner of YAP/TAZ in developmental myelination and functional nerve regeneration and as a novel transcription factor regulating Remak bundle integrity.
© 2024, Grove et al.
-
IHC
-
Neuroscience
-
Stem Cells and Developmental Biology
In Journal of Virology on 20 February 2024 by Dochnal, S., Whitford, A. L., et al.
Herpes simplex virus-1 (HSV-1) establishes a latent infection in peripheral neurons and periodically reactivates to permit transmission, which can result in clinical manifestations. Viral transactivators required for lytic infection are largely absent during latent infection, and therefore, HSV-1 relies on the co-option of neuronal host signaling pathways to initiate its gene expression. The activation of the neuronal c-Jun N-terminal kinase (JNK) cell stress pathway is central to initiating biphasic reactivation in response to multiple stimuli. However, how host factors work with JNK to stimulate the initial wave of gene expression (known as Phase I) or the progression to full Phase II reactivation remains unclear. Here, we found that c-Jun, the primary target downstream of neuronal JNK cell stress signaling, functions during reactivation but not during the JNK-mediated initiation of Phase I gene expression. Instead, c-Jun was required to transition from Phase I to full HSV-1 reactivation and was detected in viral replication compartments of reactivating neurons. Interestingly, we also identified a role for both c-Jun and enhanced neuronal stress during initial neuronal infection in promoting a more reactivation-competent form of HSV-1 latency. Therefore, c-Jun functions at multiple stages during the HSV latent infection of neurons to promote reactivation but not during the initial JNK-dependent Phase I. Importantly, by demonstrating that initial infection conditions can contribute to later reactivation abilities, this study highlights the potential for latently infected neurons to maintain a molecular scar of previous exposure to neuronal stressors.IMPORTANCEThe molecular mechanisms that regulate the reactivation of herpes simplex virus-1 (HSV-1) from latent infection are unknown. The host transcription and pioneer factor c-Jun is the main target of the JNK cell stress pathway that is known to be important in exit of HSV from latency. Surprisingly, we found that c-Jun does not act with JNK during exit from latency but instead promotes the transition to full reactivation. Moreover, c-Jun and enhanced neuronal stress during initial neuronal infection promoted a more reactivation-competent form of HSV-1 latency. c-Jun, therefore, functions at multiple stages during HSV-1 latent infection of neurons to promote reactivation. Importantly, this study contributes to a growing body of evidence that de novo HSV-1 infection conditions can modulate latent infection and impact future reactivation events, raising important questions on the clinical impact of stress during initial HSV-1 acquisition on future reactivation events and consequences.
-
WB
-
Immunology and Microbiology
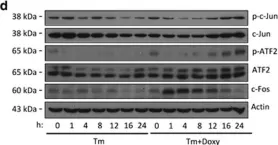
In Cell Death Dis on 6 April 2017 by Wassermann-Dozorets, R. & Rubinstein, M.
Fig.2.D

-
WB
-
Mus musculus (House mouse)
Collected and cropped from Cell Death Dis by CiteAb, provided under a CC-BY license
Image 1 of 3
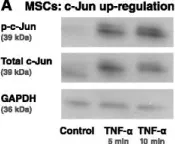
In Stem Cell Res Ther on 1 May 2015 by Katanov, C., Lerrer, S., et al.
Fig.6.A

-
WB
-
Homo sapiens (Human)
Collected and cropped from Stem Cell Res Ther by CiteAb, provided under a CC-BY license
Image 1 of 3
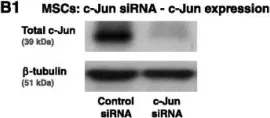
In Stem Cell Res Ther on 1 May 2015 by Katanov, C., Lerrer, S., et al.
Fig.6.B

-
WB
-
Homo sapiens (Human)
Collected and cropped from Stem Cell Res Ther by CiteAb, provided under a CC-BY license
Image 1 of 3