Small molecule imipridones including ONC201, ONC206 and ONC212 have anti-cancer activity mediated in part through the integrated stress response, induction of TRAIL and its receptor DR5, and activation of mitochondrial caseinolytic protease ClpP with impaired oxidative phosphorylation. ONC201 provides clinical benefit in a subset of patients with histone H3K27M-mutated diffuse glioma (DG). We hypothesized that EZH2 inhibitors (EZH2i) may sensitize tumors to imipridones by mimicking H3K27M mutation. EZH1 is a homolog and alternative for EZH2 in assembling PRC2 complex. We combined ONC201, ONC206 or ONC212 plus dual EZH1/2i in tumors and observed synergy. We observed synergies with imipridones combined with HDACi or triple combination of ONC201/ONC206, EZH2i and HDACi in DG, GBM, prostate cancer and SCLC cells. Our observations implicate EZH1/2 suppression in mechanism of anti-cancer effect of imipridones. We investigated effects of imipridones on EZH1/2 in DG cells and solid tumor cells including GBM, CRC, PDAC, SCLC, prostate cancer, gastric cancer, HCC and breast cancer cells and found inhibition of EZH1/EZH2 expression across tumor types and cell viability suppression by imipridones is correlated with EZH1/2 reduction. Imipridone or EZH2i-treated tumor cells showed similar cytokine profile changes. RNA-seq showed ONC201 and EHZ2i tazemetostat-treated cells have similar transcriptional profiles and share overlap of top regulated genes. Thus, imipridones inhibit EZH1/2 in tumor cells in a manner that mimics H3K27M mutation supporting their role in anti-cancer efficacy. ONC201 and EZH2i share similar targets and actions on tumors. Synergistic combinations of imipridones plus EZH1/2i or imipridones, EZH2i and HDACi merit further investigation.
AJCR Copyright © 2025.
Product Citations: 61
In American Journal of Cancer Research on 14 April 2025 by Zhang, Y., Huntington, K. E., et al.
-
Cancer Research
In Oncotarget on 27 March 2025 by Zhou, L., Zhang, L., et al.
Glioblastoma remains a lethal brain tumor in adults with limited therapeutic options. TIC10/ONC201, a first-in-class imipridone we discovered, achieved meaningful therapeutic effects in phase I/II trials in patients with diffuse gliomas (DG's) harboring H3K27M mutations, and currently the drug is in randomized phase III testing (ACTION trial; NCT05580562). ONC201 targets mitochondrial protease ClpP to disrupt oxidative phosphorylation and trigger the integrated stress response (ISR), TRAIL/DR5, and tumor cell death. While ONC201 and its analog ONC206 are undergoing clinical trials as single agents, there is limited information on their interactions with stand-of-care therapy. We show that ONC201 and ONC206 synergize with temozolomide (TMZ) and Radiotherapy (RT). ONC201 enhances TMZ- or RT-induced apoptosis, ISR and cytotoxicity. ClpP-silencing suppresses ONC201-induced cytotoxicity but not TMZ. Both ONC201 and ONC206 reduce expression of TMZ-resistance mediator MGMT observed in H3K27M-mutated DG cells following treatment with imipridones+TMZ. Cytokine profiling indicates distinct effects of ONC201 relative to TMZ treatment. These results suggest mechanisms underlying ONC201's anti-tumoral activity are distinct from those associated with TMZ or RT with potential for synergy between these three treatments. Triple ONC201+RT+TMZ (IRT) therapy prolonged median survival to 123 days with tail on survival curve (3-of-7 mice alive beyond 200-days) in orthotopic U251 GBM model versus ONC201 (44-days; p = 0.000197), RT (63-days; p = 0.0012), TMZ (78-days; p = 0.0354), ONC201+RT (55-days; p = 0.0004), ONC201+TMZ (80-days; p = 0.0041) and RT+TMZ (103-days; p > 0.05). By 231-days, the only surviving mice were in IRT group. Our results support investigation of ONC201/ONC206 in combination with RT/TMZ (IRT) in GBM or H3K27M mutated DG therapy.
-
Cancer Research
In American Journal of Cancer Research on 13 January 2025 by Wu, L. J., Pinho-Schwermann, M., et al.
Androgen receptor (AR) signaling is a target in prostate cancer therapy and can be treated with non-steroidal anti-androgens (NSAA) including enzalutamide, and apalutamide for patients with advanced disease. Metastatic castration-resistant prostate cancer (mCPRC) develop resistance becomes refractory to therapy limiting patient overall survival. Darolutamide is a novel next-generation androgen receptor-signaling inhibitor that is FDA approved for non-metastatic castration resistant prostate cancer (nmCRPC). Imipridone ONC201/TIC10 is first-in-class small molecule imipridone that activates the integrated stress response (ISR), upregulates TNF-related apoptosis-inducing ligand (TRAIL) and has activity against CRPC alone or in combination with enzalutamide in preclinical models. We hypothesized that combination of imipridones with androgen receptor signaling blockers such as darolutamide may synergize in anti-tumor efficacy against mCRPC cells. mCRPC cell lines 22RV1, LNCaP, DU145 and PC3 were treated with imipridones ONC201, ONC206, apalutamide, darolutamide, or enzalutamide as single agents or in combinations. Combinations of ONC201 or ONC206 and androgen receptor signaling blockers demonstrated synergistic effects in mCRPC cells. Combinations of ONC201 and darolutamide or enzalutamide reduced PSA levels in LNCaP cells and induced of ATF4 in both LNCaP and 22RV1 cell lines. Darolutamide synergized with ONC201 regardless of AR status or castration sensitivity in vitro. Flow cytometric analysis showed increased intra-tumoral NK cells in mice treated with ONC201 and combination of ONC201 and darolutamide. Trends of increased TRAIL activation within NK cells were also observed in treatment groups. ONC201 and darolutamide demonstrated anti-tumor effects in vivo in the 22RV1 CRPC model. Our results prompt further translational and clinical studies with imipridones ONC201 or ONC206 in combination with enzalutamide or darolutamide for treatment of castrate resistant advanced or metastatic prostate cancer.
AJCR Copyright © 2024.
-
WB
-
Cancer Research
In Acta Neuropathologica Communications on 26 December 2024 by Thompson, E. G., Spead, O., et al.
The G4C2 hexanucleotide repeat expansion in C9ORF72 is the major genetic cause of both amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD) (C9-ALS/FTD). Despite considerable efforts, the development of mouse models of C9-ALS/FTD useful for therapeutic development has proven challenging due to the intricate interplay of genetic and molecular factors underlying this neurodegenerative disorder, in addition to species differences. This study presents a robust investigation of the cellular pathophysiology and behavioral outcomes in a previously described AAV mouse model of C9-ALS expressing 66 G4C2 hexanucleotide repeats. The model displays key molecular ALS pathological markers including RNA foci, dipeptide repeat (DPR) protein aggregation, p62 positive stress granule formation as well as mild gliosis. However, the AAV-(G4C2)66 mouse model in this study has marginal neurodegeneration with negligible neuronal loss, or clinical deficits. Human C9orf72 is typically associated with altered TAR DNA-binding protein (TDP-43) function, yet studies of this rodent model revealed no significant evidence of TDP-43 dysfunction. While our findings indicate and support that this is a highly valuable robust and pharmacologically tractable model for investigating the molecular mechanisms and cellular consequences of (G4C2) repeat driven DPR pathology, it is not suitable for investigating the development of disease- associated TDP-43 dysfunction or clinical impairment. Our findings underscore the complexity of ALS pathogenesis involving genetic mutations and protein dysregulation and highlight the need for more comprehensive model systems that reliably replicate the multifaceted cellular and behavioral aspects of C9-ALS.
© 2024. The Author(s).
-
IHC-IF
-
Mus musculus (House mouse)
-
Pathology
Preprint on BioRxiv : the Preprint Server for Biology on 1 August 2024 by Wu, L., Pinho-Schwermann, M., et al.
Androgen receptor (AR) signaling plays a primary role in prostate cancer progression. Non-steroidal anti- androgens (NSAA) including enzalutamide, and apalutamide have been used to treat patients with advanced disease. However, patients with metastatic castration-resistant prostate cancer (mCPRC) develop resistance, resulting in limited overall survival benefit. Darolutamide is a novel next-generation androgen receptor- signaling inhibitor that is FDA approved for non-metastatic castration resistant prostate cancer (nmCRPC). Imipridone ONC201/TIC10 is first-in-class small molecule that activates the integrated stress response (ISR) and upregulates TNF-related apoptosis-inducing ligand (TRAIL). Our study investigates ISR and AR signaling in anti-tumor efficacy with ONC201 and enzalutamide or darolutamide against mCRPC cells. mCRPC cell lines 22RV1, LNCaP, DU145 and PC3 were treated with ONC201, darolutamide, and enzalutamide as single agents or in combinations. Combinations of ONC201 and darolutamide or enzalutamide demonstrated synergistic effects in mCRPC cells. Combinations of ONC201 and darolutamide or enzalutamide reduced PSA levels in LNCaP cells and induced of ATF4 in both LNCaP and 22RV1 cell lines. Darolutamide synergized with ONC201 regardless of AR status or castration sensitivity in vitro. Flow cytometric analysis showed increased intra-tumoral NK cells in mice treated with ONC201 and combination of ONC201 and darolutamide. Trends of increased TRAIL activation within NK cells were also observed in treatment groups. ONC201 and darolutamide demonstrated anti-tumor effects in vivo in the 22RV1 CRPC model. Our results prompt further translational and clinical studies with imipridones ONC201 or ONC201 in combination with enzalutamide or darolutamide for treatment of castrate resistant advanced or metastatic prostate cancer.
-
Cancer Research
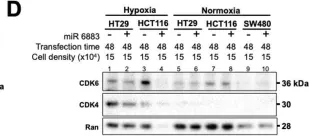
In MicroPubl Biol on 12 February 2024 by Jensen-Velez, N. A., Carlsen, L., et al.
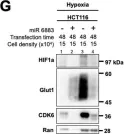
Fig.1.G

-
WB
-
Collected and cropped from MicroPubl Biol by CiteAb, provided under a CC-BY license
Image 1 of 7
In MicroPubl Biol on 12 February 2024 by Jensen-Velez, N. A., Carlsen, L., et al.
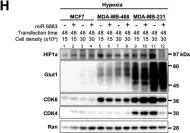
Fig.1.H

-
WB
-
Collected and cropped from MicroPubl Biol by CiteAb, provided under a CC-BY license
Image 1 of 7
In MicroPubl Biol on 12 February 2024 by Jensen-Velez, N. A., Carlsen, L., et al.
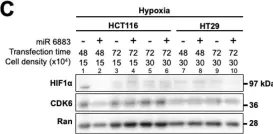
Fig.1.C

-
WB
-
Collected and cropped from MicroPubl Biol by CiteAb, provided under a CC-BY license
Image 1 of 7
In MicroPubl Biol on 12 February 2024 by Jensen-Velez, N. A., Carlsen, L., et al.
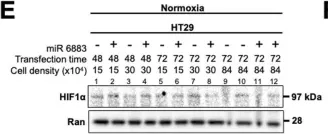
Fig.1.E

-
WB
-
Collected and cropped from MicroPubl Biol by CiteAb, provided under a CC-BY license
Image 1 of 7
In MicroPubl Biol on 12 February 2024 by Jensen-Velez, N. A., Carlsen, L., et al.
Fig.1.D

-
WB
-
Collected and cropped from MicroPubl Biol by CiteAb, provided under a CC-BY license
Image 1 of 7
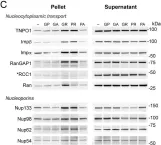
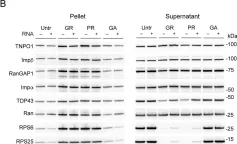
In Elife on 2 March 2020 by Hayes, L. R., Duan, L., et al.
Fig.4.C

-
WB
-
Collected and cropped from Elife by CiteAb, provided under a CC-BY license
Image 1 of 7
In Elife on 2 March 2020 by Hayes, L. R., Duan, L., et al.
Fig.5.B

-
WB
-
Collected and cropped from Elife by CiteAb, provided under a CC-BY license
Image 1 of 7