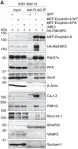
A missense mutation in the transcription repressor Nucleus accumbens-associated 1 (NACC1) gene at c.892C>T (p.Arg298Trp) on chromosome 19 causes severe neurodevelopmental delay ( Schoch et al., 2017). To model this disorder, we engineered the first mouse model with the homologous mutation (Nacc1+/R284W ) and examined mice from E17.5 to 8 months. Both genders had delayed weight gain, epileptiform discharges and altered power spectral distribution in cortical electroencephalogram, behavioral seizures, and marked hindlimb clasping; females displayed thigmotaxis in an open field. In the cortex, NACC1 long isoform, which harbors the mutation, increased from 3 to 6 months, whereas the short isoform, which is not present in humans and lacks aaR284 in mice, rose steadily from postnatal day (P) 7. Nuclear NACC1 immunoreactivity increased in cortical pyramidal neurons and parvalbumin containing interneurons but not in nuclei of astrocytes or oligodendroglia. Glial fibrillary acidic protein staining in astrocytic processes was diminished. RNA-seq of P14 mutant mice cortex revealed over 1,000 differentially expressed genes (DEGs). Glial transcripts were downregulated and synaptic genes upregulated. Top gene ontology terms from upregulated DEGs relate to postsynapse and ion channel function, while downregulated DEGs enriched for terms relating to metabolic function, mitochondria, and ribosomes. Levels of synaptic proteins were changed, but number and length of synaptic contacts were unaltered at 3 months. Homozygosity worsened some phenotypes including postnatal survival, weight gain delay, and increase in nuclear NACC1. This mouse model simulates a rare form of autism and will be indispensable for assessing pathophysiology and targets for therapeutic intervention.
Copyright © 2024 the authors.
Product Citations: 17
Nacc1 Mutation in Mice Models Rare Neurodevelopmental Disorder with Underlying Synaptic Dysfunction.
In The Journal of Neuroscience on 3 April 2024 by Deehan, M. A., Kothuis, J. M., et al.
-
Mus musculus (House mouse)
-
Neuroscience
Psychoneurobiology Research and Personalized Treatment of Schizophrenia.
In Journal of Personalized Medicine on 7 December 2021 by Sumiyoshi, T.
In Journal of Personalized Medicine on 26 June 2021 by Nitta, A., Izuo, N., et al.
Piccolo, a presynaptic cytomatrix protein, plays a role in synaptic vesicle trafficking in the presynaptic active zone. Certain single-nucleotide polymorphisms of the Piccolo-encoding gene PCLO are reported to be associated with mental disorders. However, a few studies have evaluated the relationship between Piccolo dysfunction and psychotic symptoms. Therefore, we investigated the neurophysiological and behavioral phenotypes in mice with Piccolo suppression in the medial prefrontal cortex (mPFC). Downregulation of Piccolo in the mPFC reduced regional synaptic proteins, accompanied with electrophysiological impairments. The Piccolo-suppressed mice showed an enhanced locomotor activity, impaired auditory prepulse inhibition, and cognitive dysfunction. These abnormal behaviors were partially ameliorated by the antipsychotic drug risperidone. Piccolo-suppressed mice received mild social defeat stress showed additional behavioral despair. Furthermore, the responses of these mice to extracellular glutamate and dopamine levels induced by the optical activation of mPFC projection in the dorsal striatum (dSTR) were inhibited. Similarly, the Piccolo-suppressed mice showed decreased depolarization-evoked glutamate and -aminobutyric acid elevations and increased depolarization-evoked dopamine elevation in the dSTR. These suggest that Piccolo regulates neurotransmission at the synaptic terminal of the projection site. Reduced neuronal connectivity in the mPFC-dSTR pathway via suppression of Piccolo in the mPFC may induce behavioral impairments observed in schizophrenia.
-
WB
-
Mus musculus (House mouse)
In Frontiers in Synaptic Neuroscience on 6 April 2021 by Iuliano, M., Seeley, C., et al.
Dysfunction at synapses is thought to be an early change contributing to cognitive, psychiatric and motor disturbances in Huntington's disease (HD). In neurons, mutant Huntingtin collects in aggregates and distributes to the same sites as wild-type Huntingtin including on membranes and in synapses. In this study, we investigated the biochemical integrity of synapses in HD mouse striatum. We performed subcellular fractionation of striatal tissue from 2 and 6-month old knock-in Q175/Q7 HD and Q7/Q7 mice. Compared to striata of Q7/Q7 mice, proteins including GLUT3, Na+/K+ ATPase, NMDAR 2b, PSD95, and VGLUT1 had altered distribution in Q175/Q7 HD striata of 6-month old mice but not 2-month old mice. These proteins are found on plasma membranes and pre- and postsynaptic membranes supporting hypotheses that functional changes at synapses contribute to cognitive and behavioral symptoms of HD. Lipidomic analysis of mouse fractions indicated that compared to those of wild-type, fractions 1 and 2 of 6 months Q175/Q7 HD had altered levels of two species of PIP2, a phospholipid involved in synaptic signaling, increased levels of cholesterol ester and decreased cardiolipin species. At 2 months, increased levels of species of acylcarnitine, phosphatidic acid and sphingomyelin were measured. EM analysis showed that the contents of fractions 1 and 2 of Q7/Q7 and Q175/Q7 HD striata had a mix of isolated synaptic vesicles, vesicle filled axon terminals singly or in clusters, and ER and endosome-like membranes. However, those of Q175/Q7 striata contained significantly fewer and larger clumps of particles compared to those of Q7/Q7. Human HD postmortem putamen showed differences from control putamen in subcellular distribution of two proteins (Calnexin and GLUT3). Our biochemical, lipidomic and EM analysis show that the presence of the HD mutation conferred age dependent disruption of localization of synaptic proteins and lipids important for synaptic function. Our data demonstrate concrete biochemical changes suggesting altered integrity of synaptic compartments in HD mice that may mirror changes in HD patients and presage cognitive and psychiatric changes that occur in premanifest HD.
Copyright © 2021 Iuliano, Seeley, Sapp, Jones, Martin, Li, DiFiglia and Kegel-Gleason.
In Alzheimer's Dementia : the Journal of the Alzheimer's Association on 1 September 2019 by Liu, L., Lauro, B. M., et al.
There is keen interest in elucidating the biological mechanisms underlying recent failures of β-site amyloid precursor protein-cleaving enzyme-1 (BACE1) inhibitors in Alzheimer's disease trials.
We developed a highly sensitive and specific immunoassay for BACE1 in cell lines and iPSC-derived human neurons to systematically analyze the effects of eight clinically relevant BACE1 inhibitors.
Seven of 8 inhibitors elevated BACE1 protein levels. Among protease inhibitors tested, the elevation was specific to BACE1 inhibitors. The inhibitors did not increase BACE1 transcription but extended the protein's half-life. BACE1 became elevated at concentrations below the IC50 for amyloid β (Aβ).
Elevation of BACE1 by 7 of 8 BACE1 inhibitors raises new concerns about advancing such β-secretase inhibitors for AD. Chronic elevation could lead to intermittently uninhibited BACE1 when orally dosed inhibitors reach trough levels, abnormally increasing substrate processing. Compounds such as roburic acid that lower Aβ by dissociating β/γ secretase complexes are better candidates because they neither inhibit β- and γ-secretase nor increase BACE1 levels.
Copyright © 2019 the Alzheimer's Association. Published by Elsevier Inc. All rights reserved.
-
WB
-
Neuroscience
In Elife on 4 July 2017 by Fan, F., Matsunaga, K., et al.
Fig.8.A

-
WB
-
Rattus norvegicus (Rat)
Collected and cropped from Elife by CiteAb, provided under a CC-BY license
Image 1 of 1