Resection surgery is the first-line therapy for glioblastoma (GBM) that is performed in >70% of patients, typically within days of suspected diagnosis. Current protocols for follow-on chemoradiotherapy have shown only modest efficacy in eliminating residual disease, leading to inevitable tumour recurrence. There remains a need for new approaches to swiftly and effectively treat post-operative residual disease to prevent the rapid early progression of recurrent GBM. Using syngeneic preclinical models of glioblastoma resection, we identified a spatially and temporally restricted window of blood brain barrier (BBB) disruption localised to the resection margin, during the immediate (15 min) and early (48-72h) postoperative periods. Intravenous administration of fluorescently labelled, clinically-used liposome nanoparticles during these periods demonstrated that selective accumulation at the postoperative resection margin, while largely being excluded from areas of the brain with an intact BBB, could be achieved. Confocal analysis confirmed the presence of extravasated nanoparticles within the margin parenchyma which largely interacted with microglial populations closely associated with residual tumour cells. Exploiting this, we performed intravenous administration of doxorubicin-loaded liposomes (DOX-Lipo) coinciding with the peak of postoperative BBB disruption and demonstrated both enhanced chemotherapy delivery and consequently complete inhibition of tumour recurrence from a single administration. Overall, this work underscores the importance of timing concomitant chemotherapy to the post-operative timeframe and demonstrates that clinically-used liposomal nanomedicines could be readily repurposed for early post-operative therapy in aggressive brain tumours.
Product Citations: 76
Preprint on BioRxiv : the Preprint Server for Biology on 3 April 2025 by Fernandes, L. F., Peeyatu, C., et al.
-
Cardiovascular biology
In Cell Communication and Signaling : CCS on 17 March 2025 by Huilcaman, R., Campos, A., et al.
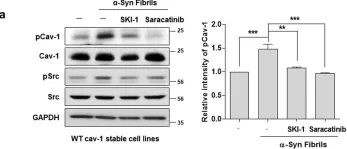
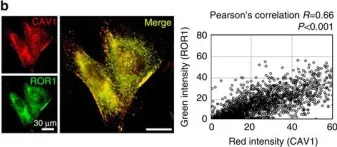
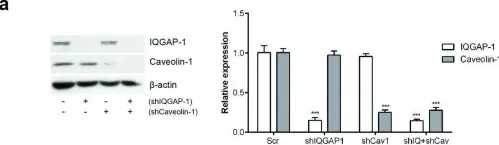
Caveolin-1 (CAV1) is a membrane protein that promotes migration, invasion and metastasis of cancer cells when phosphorylated on tyrosine-14 (Y14) by a cell intrinsic mechanism involving the activation of a novel Rab5-Rac1 signaling axis. Moreover, CAV1 expressed in aggressive cancer cells is included into extracellular vesicles (EVs) and such EVs increase the metastatic potential of recipient lower grade cancer cells. However, the relevance of CAV1 Y14 phosphorylation in these extrinsic EV-stimulated events remained to be determined. Here we used B16F10 mouse melanoma cells over-expressing wild-type CAV1, phospho-mimetic CAV1(Y14E) or phospho-null CAV1(Y14F) as models to determine how the EV protein content was affected by Y14 phosphorylation and how these EVs modulated the metastatic potential of recipient B16F10 cells lacking CAV1. EVs from B16F10 cells over-expressing wild-type and CAV1(Y14/E) contain CAV1, and other proteins linked to signaling pathways associated with cell adhesion and migration. CAV1 inclusion in EVs was reduced by the Y14F mutation and global protein composition was also significantly different. Moreover, CAV1 wild-type and CAV1(Y14E) EVs promoted migration, as well as invasion of cells lacking CAV1 [B16F10(Mock) cells]. In addition, β3 integrin was transferred via CAV1(Y14E) EVs to B16F10 (Mock) cells, and treatment with such EVs promoted metastasis of recipient B16F10(Mock) cells. Finally, CAV1(Y14E) EV-enhanced migration, invasion and metastasis of recipient cells was blocked by anti-αVβ3 antibodies. In conclusion, CAV1 phosphorylated on Y14 not only intrinsically promotes migration, invasion and metastasis of cells expressing the protein (in cis), but also favors the inclusion of CAV1 into EVs, as well as the extrinsic acquisition of malignant traits in recipient cells, through integrin transfer (in trans).
© 2025. The Author(s).
-
WB
-
Cancer Research
-
Endocrinology and Physiology
In Development (Cambridge, England) on 1 December 2024 by Wang, Z. Y., Mehra, A., et al.
VEGFA administration has been explored as a pro-angiogenic therapy for cardiovascular diseases including heart failure for several years, but with little success. Here, we investigate a different approach to augment VEGFA bioavailability: by deleting the VEGFA decoy receptor VEGFR1 (also known as FLT1), one can achieve more physiological VEGFA concentrations. We find that after cryoinjury, zebrafish flt1 mutant hearts display enhanced coronary revascularization and endocardial expansion, increased cardiomyocyte dedifferentiation and proliferation, and decreased scarring. Suppressing Vegfa signaling in flt1 mutants abrogates these beneficial effects of flt1 deletion. Transcriptomic analyses of cryoinjured flt1 mutant hearts reveal enhanced endothelial MAPK/ERK signaling and downregulation of the transcription factor gene egr3. Using newly generated genetic tools, we observe egr3 upregulation in the regenerating endocardium, and find that Egr3 promotes myofibroblast differentiation. These data indicate that with enhanced Vegfa bioavailability, the endocardium limits myofibroblast differentiation via egr3 downregulation, thereby providing a more permissive microenvironment for cardiomyocyte replenishment after injury.
© 2024. Published by The Company of Biologists Ltd.
-
Cardiovascular biology
-
Stem Cells and Developmental Biology
In Biology Open on 15 November 2024 by Terada, Y., Obara, K., et al.
Exosomes are small extracellular vesicles (sEVs) secreted via multivesicular bodies (MVBs)/late endosomes and mediators of cell-cell communication. We previously reported a novel post-translational modification by ubiquitin-like 3 (UBL3). UBL3 is localized in MVBs and the plasma membrane and released outside as sEVs, including exosomes. Approximately 60% of proteins sorted in sEVs are affected by UBL3 and localized in various organelles, the plasma membrane, and the cytosol, suggesting that its dynamic movement in the cell before entering the MVBs. To examine the intracellular dynamics of UBL3, we constructed a sophisticated visualization system via fusing fluorescent timers that changed from blue to red form over time with UBL3 and by its expression under Tet-on regulation. Intriguingly, we found that after synthesis, UBL3 was initially distributed within the cytosol. Subsequently, UBL3 was localized to MVBs and the plasma membrane and finally showed predominant accumulation in MVBs. Furthermore, by super-resolution microscopy analysis, UBL3 was found to be associated with one of its substrates, α-tubulin, in the cytosol, and the complex was subsequently transported to MVBs. This spatiotemporal visualization system for UBL3 will form a basis for further studies to elucidate when and where UBL3 associates with its substrates/binding proteins before localization in MVBs.
© 2024. Published by The Company of Biologists Ltd.
Membrane tension regulation is required for wound repair
Preprint on BioRxiv : the Preprint Server for Biology on 16 August 2024 by Raj, N., Weiss, M., et al.
Disruptions of the eukaryotic plasma membrane due to chemical and mechanical challenges are frequent and detrimental, and thus need to be repaired to maintain proper cell function and avoid cell death. However, the cellular mechanisms involved in wound resealing and restoration of homeostasis are diverse and contended. Here, we show that clathrin-mediated endocytosis is induced at later stages of plasma membrane wound repair following the actual resealing of the wound. This compensatory endocytosis occurs near the wound, predominantly at sites of previous early endosome exocytosis which is required in the initial stage of membrane resealing, suggesting a spatio-temporal co-ordination of exo- and endocytosis during wound repair. Using cytoskeletal alterations and modulation of membrane tension and membrane area, we identify membrane tension as a major regulator of the wounding-associated exo- and endocytic events that mediate efficient wound repair. Thus, membrane tension changes are a universal trigger for plasma membrane wound repair modulating the exocytosis of early endosomes required for resealing and subsequent clathrin-mediated endocytosis acting at later stages to restore cell homeostasis and function.
-
Homo sapiens (Human)
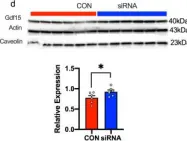
In Nat Commun on 30 August 2022 by Zapata, R. C., Carretero, M., et al.
Fig.8.D

-
WB
-
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 20
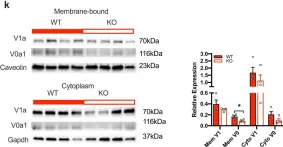
In Nat Commun on 30 August 2022 by Zapata, R. C., Carretero, M., et al.
Fig.4.K

-
WB
-
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 20
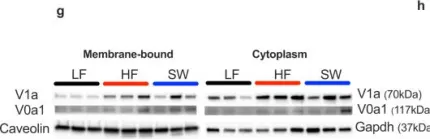
In Nat Commun on 30 August 2022 by Zapata, R. C., Carretero, M., et al.
Fig.2.G

-
WB
-
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 20
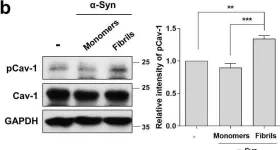
In Mol Brain on 28 July 2021 by Ha, T. Y., Choi, Y. R., et al.
Fig.4.A

-
WB
-
Collected and cropped from Mol Brain by CiteAb, provided under a CC-BY license
Image 1 of 20
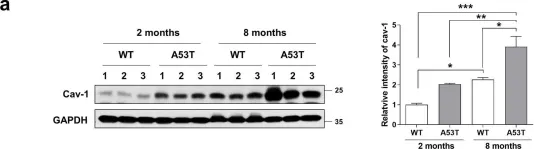
In Mol Brain on 28 July 2021 by Ha, T. Y., Choi, Y. R., et al.
Fig.3.B

-
WB
-
Collected and cropped from Mol Brain by CiteAb, provided under a CC-BY license
Image 1 of 20
In Mol Brain on 28 July 2021 by Ha, T. Y., Choi, Y. R., et al.
Fig.1.A

-
WB
-
Collected and cropped from Mol Brain by CiteAb, provided under a CC-BY license
Image 1 of 20
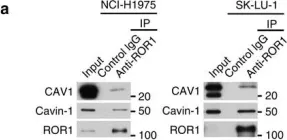
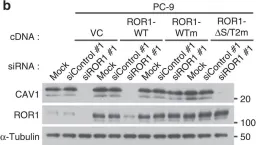
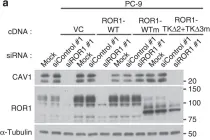
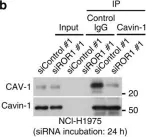
In Nat Commun on 4 January 2016 by Yamaguchi, T., Lu, C., et al.
Fig.3.D

-
WB
-
Homo sapiens (Human)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 20
In Nat Commun on 4 January 2016 by Yamaguchi, T., Lu, C., et al.
Fig.5.C

-
WB
-
Homo sapiens (Human)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 20
In Nat Commun on 4 January 2016 by Yamaguchi, T., Lu, C., et al.
Fig.5.E

-
WB
-
Homo sapiens (Human)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 20
In Nat Commun on 4 January 2016 by Yamaguchi, T., Lu, C., et al.
Fig.5.A

-
WB
-
Homo sapiens (Human)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 20
In Nat Commun on 4 January 2016 by Yamaguchi, T., Lu, C., et al.
Fig.6.B

-
WB
-
Homo sapiens (Human)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 20
In Nat Commun on 4 January 2016 by Yamaguchi, T., Lu, C., et al.
Fig.6.A

-
WB
-
Homo sapiens (Human)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 20
In Nat Commun on 4 January 2016 by Yamaguchi, T., Lu, C., et al.
Fig.7.B

-
WB
-
Homo sapiens (Human)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 20
In Nat Commun on 4 January 2016 by Yamaguchi, T., Lu, C., et al.
Fig.7.A

-
WB
-
Homo sapiens (Human)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 20
In Nat Commun on 4 January 2016 by Yamaguchi, T., Lu, C., et al.
Fig.2.C

-
WB
-
Homo sapiens (Human)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 20
In Nat Commun on 4 January 2016 by Yamaguchi, T., Lu, C., et al.
Fig.2.A

-
WB
-
Homo sapiens (Human)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 20
In Nat Commun on 4 January 2016 by Yamaguchi, T., Lu, C., et al.
Fig.3.C

-
ICC-IF
-
Homo sapiens (Human)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 20
In Nat Commun on 4 January 2016 by Yamaguchi, T., Lu, C., et al.
Fig.3.B

-
ICC-IF
-
Homo sapiens (Human)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 20
In Nat Commun on 4 January 2016 by Yamaguchi, T., Lu, C., et al.
Fig.3.A

-
WB
-
Homo sapiens (Human)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 20
In Oncotarget on 10 April 2015 by Moon, H., Ruelcke, J. E., et al.
Fig.5.A

-
WB
-
Collected and cropped from Oncotarget by CiteAb, provided under a CC-BY license
Image 1 of 20