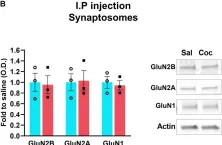
Phenylketonuria (PKU), an inborn error of phenylalanine (Phe) metabolism, is a common cause of intellectual disability. However, the mechanisms by which elevated phenylalanine (Phe) levels cause cognitive impairment remain unclear. Here, we show that submillimolar Phe perturbs synaptic plasticity through the hyperactivation of GluN2B-containing NMDARs. PahEnu2 PKU model mice exhibited submillimolar and supramillimolar concentrations of Phe in the cerebrospinal fluid (CSF) and serum, respectively. L-Phe produced concentration-dependent bidirectional effects on NMDA-induced currents, without affecting synaptic NMDARs in hippocampal CA1 neurons. L-Phe-induced hyperactivation of extrasynaptic GluN2B resulted in activity-dependent downregulation of AMPARs during burst or sustained synaptic activity. Administration of L-Phe in mice decreased neural activity and impaired memory, which were blocked by pretreatment with GluN2B inhibitors. Furthermore, pharmacological and virus-mediated suppression of GluN2B reversed the impaired learning in PahEnu2 mice. Collectively, these results suggest that the concentration of Phe in the CSF of patients with PKU perturbs extrasynaptic NMDARs and synaptic plasticity, and that suppression of GluN2B may have the potential to improve cognitive function in patients with PKU.
Product Citations: 52
In The Journal of Clinical Investigation on 8 May 2025 by Song, W. S., Kim, Y. S., et al.
-
Neuroscience
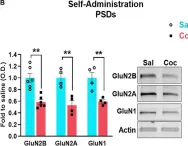
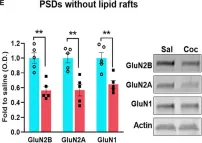
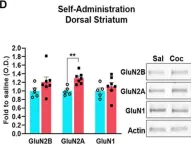
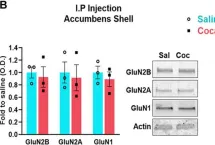
GluN2B-mediated regulation of silent synapses for receptor specification and addiction memory.
In Experimental & Molecular Medicine on 1 February 2025 by Kim, H. J., Lee, S., et al.
Psychostimulants, including cocaine, elicit stereotyped, addictive behaviors. The reemergence of silent synapses containing only NMDA-type glutamate receptors is a critical mediator of addiction memory and seeking behaviors. Despite the predominant abundance of GluN2B-containing NMDA-type glutamate receptors in silent synapses, their operational mechanisms are not fully understood. Here, using conditional depletion/deletion of GluN2B in D1-expressing accumbal medium spiny neurons, we examined the synaptic and behavioral actions that silent synapses incur after repeated exposure to cocaine. GluN2B ablation reduces the proportion of silent synapses, but some of them can persist by substitution with GluN2C, which drives the aberrantly facilitated synaptic incorporation of calcium-impermeable AMPA-type glutamate receptors (AMPARs). The resulting precocious maturation of silent synapses impairs addiction memory but increases locomotor activity, both of which can be normalized by the blockade of calcium-impermeable AMPAR trafficking. Collectively, GluN2B supports the competence of cocaine-induced silent synapses to specify the subunit composition of AMPARs and thereby the expression of addiction memory and related behaviors.
© 2025. The Author(s).
-
Biochemistry and Molecular biology
-
Neuroscience
In Frontiers in Pharmacology on 21 January 2025 by Zelek-Molik, A., Gądek-Michalska, A., et al.
Stress-evoked dysfunctions of the frontal cortex (FC) are correlated with changes in the functioning of the glutamatergic system, and evidence demonstrates that noradrenergic transmission is an important regulator of this process. In the current study, we adopted a restraint stress (RS) model in male Wistar rats to investigate whether the blockade of β1 adrenergic receptors (β1AR) with betaxolol (BET) in stressed animals influences the body's stress response and the expression of selected signaling proteins in the medial prefrontal cortex (mPFC).
The study was divided into two parts. In the first part, rats were exposed to RS for 3, 7, or 14 days, and the expression of glutamate signaling proteins (p(S845)/t GluA1, p(Y1472)/t GluN2B, VGLUT1, and VGLUT2) in the FC was analyzed to determine the optimal RS duration for studying the mechanisms of hypofrontality. In the second part, rats were exposed to RS for 14 days, and BET (5 mg/kg, p. o.) was administered during the last 8 days immediately after RS. The body's stress reaction was assessed by analyzing body weight and blood levels of adrenocorticotropic hormone (ACTH) and corticosterone (CORT). Behavioral responses were evaluated using the novel object recognition (NOR) and elevated plus maze (EPM) tests. The impact of RS and BET on the expression of p(Y530)/t Fyn and p (S133)/t CREB in the mPFC was measured via Western blotting.
The first part of the study demonstrated a decreased level of glutamate receptors in rats exposed to 14 days of RS, following an initial increase observed after 7 days of RS. Results from the second part revealed that chronic RS reduced body weight, impaired recognition memory in the NOR test, augmented blood levels of ACTH, and increased the expression of p(Y530) Fyn in the mPFC. However, β1AR blockade did not alter the effects of RS on weight gain, cognitive function, or the expression of p(Y530) Fyn. β1AR blockade normalized only the blood concentration of ACTH. These results suggest that decreased Fyn kinase activity, indicated by phosphorylation at Y530, underlies the stress-evoked downregulation of GluN2B in the FC in a manner independent of β1AR activity.
Copyright © 2025 Zelek-Molik, Gądek-Michalska, Wilczkowski, Bielawski, Maziarz, Kreiner and Nalepa.
-
WB
-
Rattus norvegicus (Rat)
-
Pharmacology
GluN2B-mediated regulation of silent synapses for receptor specification and addiction memory
Preprint on BioRxiv : the Preprint Server for Biology on 20 May 2024 by Kim, H. J., Lee, S., et al.
Psycho-stimulants including cocaine elicit stereotyped, addictive behaviors. Re-emergence of silent synapses containing only NMDA-type glutamate receptors (NMDARs) is a critical mediator of addiction memory and seeking behaviors. Despite the predominant abundance of GluN2B-containing NMDARs in silent synapses, their operational mechanisms are not fully understood. Using conditional depletion/deletion of GluN2B at D1-expressing accumbal medium spiny neurons, we examine the synaptic and behavioral actions that silent synapses incur after repeated exposure to cocaine. GluN2B ablation reduces the proportion of silent synapses, but some of them can persist by substitution to GluN2C, which drives the aberrantly-facilitated synaptic incorporation of calcium-impermeable AMPA-type glutamate receptors (AMPARs). The resultant precocious maturation of silent synapses impairs addiction memory but elevates locomotor activity, which can be normalized by blockade of calcium-impermeable AMPAR trafficking. Collectively, GluN2B supports the competence of cocaine-induced silent synapses for specifying subunit composition of AMPARs, and thereby expression of addition memory and the related behaviors.
-
WB
-
Mus musculus (House mouse)
-
Neuroscience
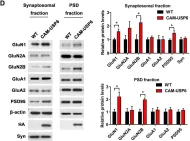
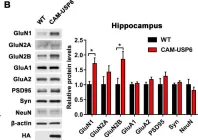
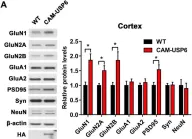
SFRP1 upregulation causes hippocampal synaptic dysfunction and memory impairment
Preprint on BioRxiv : the Preprint Server for Biology on 4 April 2024 by Pereyra, G., Mateo, M. I., et al.
Decreased dendritic complexity and impaired synaptic function are strongly linked to cognitive decline in Alzheimer’s disease (AD), and precede the emergence of other neuropathological traits that establish a harmful cycle exacerbating synaptic dysfunction. SFRP1, a glial-derived protein regulating cell-cell communication, is abnormally elevated in the brain of AD patients and related mouse models already at early disease stages. Neutralization of SFRP1 activity in mice reduces the occurrence of protein aggregates, neuroinflammation and prevents the loss of synaptic long-term potentiation (LTP). In this study, we generated transgenic mice that overexpress Sfrp1 in astrocytes to investigate whether LTP loss is due to an early influence of SFRP1 on synaptic function or results from other alterations driving disease progression. We report that SFRP1-overexpressing mice show reduced dendritic complexity and spine density in dentate gyrus granule cells during early adulthood, prior to a significant deficit in LTP response and late onset cognitive impairment. Ultrastructural analysis revealed the loss of small-sized synapses and presynaptic alterations in transgenic mice. Analysis of proteomic changes points to a general decrease in protein synthesis and modifications in the synaptic proteome, particularly of proteins related to synaptic vesicle cycle and synaptic organizers, like neurexin and neuroligin. We propose a model wherein SFRP1 directly impacts on synaptic function, by increasing the availability of synaptic organizing molecules at the synapse. These observations, combined with documented SFRP1 effects on APP processing and microglial activation, imply that SFRP1 contributes to multiple pathological effects in AD, emerging as a promising therapeutic target for this devastating disease.
-
Mus musculus (House mouse)
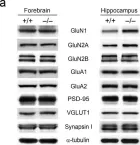
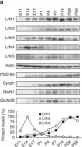
In Exp Mol Med on 1 August 2022 by Bae, Y. S., Yoon, S. H., et al.
Fig.1.A

-
WB
-
Mus musculus (House mouse)
Collected and cropped from Exp Mol Med by CiteAb, provided under a CC-BY license
Image 1 of 16
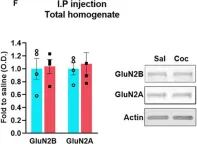
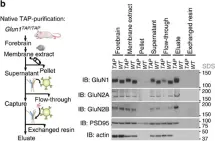
In Front Neurosci on 8 August 2020 by Delint-Ramirez, I., Segev, A., et al.
Fig.1.F

-
WB
-
Collected and cropped from Front Neurosci by CiteAb, provided under a CC-BY license
Image 1 of 16
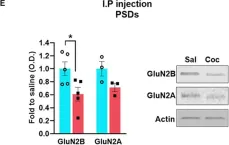
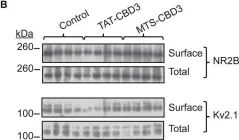
In Front Neurosci on 8 August 2020 by Delint-Ramirez, I., Segev, A., et al.
Fig.1.E

-
WB
-
Collected and cropped from Front Neurosci by CiteAb, provided under a CC-BY license
Image 1 of 16
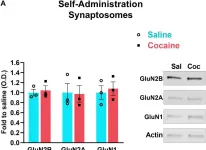
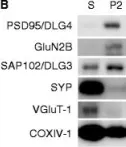
In Front Neurosci on 8 August 2020 by Delint-Ramirez, I., Segev, A., et al.
Fig.2.A

-
WB
-
Collected and cropped from Front Neurosci by CiteAb, provided under a CC-BY license
Image 1 of 16
In Front Neurosci on 8 August 2020 by Delint-Ramirez, I., Segev, A., et al.
Fig.2.B

-
WB
-
Collected and cropped from Front Neurosci by CiteAb, provided under a CC-BY license
Image 1 of 16
In Front Neurosci on 8 August 2020 by Delint-Ramirez, I., Segev, A., et al.
Fig.3.B

-
WB
-
Collected and cropped from Front Neurosci by CiteAb, provided under a CC-BY license
Image 1 of 16
In Front Neurosci on 8 August 2020 by Delint-Ramirez, I., Segev, A., et al.
Fig.3.E

-
WB
-
Collected and cropped from Front Neurosci by CiteAb, provided under a CC-BY license
Image 1 of 16
In Front Neurosci on 8 August 2020 by Delint-Ramirez, I., Segev, A., et al.
Fig.4.D

-
WB
-
Collected and cropped from Front Neurosci by CiteAb, provided under a CC-BY license
Image 1 of 16
In Front Neurosci on 8 August 2020 by Delint-Ramirez, I., Segev, A., et al.
Fig.4.B

-
WB
-
Collected and cropped from Front Neurosci by CiteAb, provided under a CC-BY license
Image 1 of 16
In PLoS Biol on 1 December 2019 by Zeng, F., Ma, X., et al.
Fig.5.D

-
WB
-
Collected and cropped from PLoS Biol by CiteAb, provided under a CC-BY license
Image 1 of 16
In PLoS Biol on 1 December 2019 by Zeng, F., Ma, X., et al.
Fig.5.B

-
WB
-
Collected and cropped from PLoS Biol by CiteAb, provided under a CC-BY license
Image 1 of 16
In PLoS Biol on 1 December 2019 by Zeng, F., Ma, X., et al.
Fig.5.A

-
WB
-
Collected and cropped from PLoS Biol by CiteAb, provided under a CC-BY license
Image 1 of 16
In Nat Commun on 12 June 2017 by Morimura, N., Yasuda, H., et al.
Fig.1.A

-
WB
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 16
In Nat Commun on 27 April 2016 by Frank, R. A., Komiyama, N. H., et al.
Fig.2.B

-
WB
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 16
In Front Cell Neurosci on 13 February 2015 by Moutal, A., François-Moutal, L., et al.
Fig.7.B

-
WB
-
Collected and cropped from Front Cell Neurosci by CiteAb, provided under a CC-BY license
Image 1 of 16
In Mol Brain on 28 November 2014 by Bayés, A., Collins, M. O., et al.
Fig.1.B

-
WB
-
Collected and cropped from Mol Brain by CiteAb, provided under a CC-BY license
Image 1 of 16