GluN2A is a NMDA receptor subunit postulated as important for learning and memory. In humans, heterozygous loss of function variants in the gene encoding it (GRIN2A) increase the risk of epilepsy, intellectual disability and schizophrenia. Haploinsufficient mouse models show electrophysiological abnormalities and thus to improve and widen understanding of the pathogenesis of GRIN2A-associated disorders in humans, this study aimed to assess the impact of Grin2a absence and haploinsufficiency on core neuronal and synaptic properties in genetically modified rats. Electrophysiological whole-cell current- and voltage-clamp recordings were made from CA1 pyramidal neurons in acute hippocampal slices from wild-type and Grin2a heterozygous (Grin2a+/- ) and homozygous (Grin2a-/- ) knock out rats aged postnatal day 27-34. While reduced levels or absence of GluN2A did not affect neuronal excitability or intrinsic membrane properties in both Grin2a+/- and Grin2a-/- rats, we found a reduced frequency of miniature excitatory post synaptic currents and a reduced density of proximal dendrites suggestive of a reduced number of excitatory synapses. Recordings from CA1 neurons in slices prepared from Grin2a+/- and Grin2a-/- rats revealed there was a reduced ratio of the current mediated by NMDA receptors compared to AMPA receptors, while in Grin2a-/- recordings, there was a slowing of the decay time-constant of the NMDA receptor-mediated excitatory postsynaptic currents. Moreover, neither summation of sub-threshold excitatory postsynaptic potentials nor summation of supra-threshold excitatory postsynaptic potentials to initiate action potential firing in CA1 pyramidal neurons indicated any dependence on GluN2A. We conclude that reduced levels of GluN2A alters the kinetics of NMDA receptor-mediated synaptic events and dendritic structure of CA1 neurons, but do not affect several other core neuronal functions. These relatively subtle changes are consistent with the largely intact neural functioning of the majority of humans carrying GRIN2A loss of function variants. Further research could explore whether the changes in synaptic properties we observed contribute to alterations in higher level circuit dynamics and computation, which may manifest as disorders of cognition and excitability in humans.
© The Author(s) 2025. Published by Oxford University Press on behalf of the Guarantors of Brain.
Product Citations: 25
In Brain Communications on 14 April 2025 by Yasmin, F., Marwick, K. F. M., et al.
-
WB
-
Immunology and Microbiology
-
Neuroscience
Maf1 loss regulates spinogenesis and attenuates cognitive impairment in Alzheimer's disease.
In Brain on 3 June 2024 by Han, Y., Chen, K., et al.
Alzheimer's disease is neurodegenerative and characterized by progressive cognitive impairment. Synaptic dysfunction appears in the early stage of Alzheimer's disease and is significantly correlated with cognitive impairment. However, the specific regulatory mechanism remains unclear. Here, we found the transcription factor Maf1 to be upregulated in Alzheimer's disease and determined that conditional knockout of Maf1 in a transgenic mouse model of Alzheimer's disease restored learning and memory function; the downregulation of Maf1 reduced the intraneuronal calcium concentration and restored neuronal synaptic morphology. We also demonstrated that Maf1 regulated the expression of NMDAR1 by binding to the promoter region of Grin1, further regulating calcium homeostasis and synaptic remodelling in neurons. Our results clarify the important role and mechanism of the Maf1-NMDAR1 signalling pathway in stabilizing synaptic structure, neuronal function and behaviour during Alzheimer's disease pathogenesis. This therefore serves as a potential diagnostic and therapeutic target for the early stage of Alzheimer's disease.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Guarantors of Brain.
-
Mus musculus (House mouse)
-
Neuroscience
Inhibitory and excitatory synaptic neuroadaptations in the diazepam tolerant brain.
In Neurobiology of Disease on 1 September 2023 by Lorenz-Guertin, J. M., Povysheva, N., et al.
Benzodiazepine (BZ) drugs treat seizures, anxiety, insomnia, and alcohol withdrawal by potentiating γ2 subunit containing GABA type A receptors (GABAARs). BZ clinical use is hampered by tolerance and withdrawal symptoms including heightened seizure susceptibility, panic, and sleep disturbances. Here, we investigated inhibitory GABAergic and excitatory glutamatergic plasticity in mice tolerant to benzodiazepine sedation. Repeated diazepam (DZP) treatment diminished sedative effects and decreased DZP potentiation of GABAAR synaptic currents without impacting overall synaptic inhibition. While DZP did not alter γ2-GABAAR subunit composition, there was a redistribution of extrasynaptic GABAARs to synapses, resulting in higher levels of synaptic BZ-insensitive α4-containing GABAARs and a concomitant reduction in tonic inhibition. Conversely, excitatory glutamatergic synaptic transmission was increased, and NMDAR subunits were upregulated at synaptic and total protein levels. Quantitative proteomics further revealed cortex neuroadaptations of key pro-excitatory mediators and synaptic plasticity pathways highlighted by Ca2+/calmodulin-dependent protein kinase II (CAMKII), MAPK, and PKC signaling. Thus, reduced inhibitory GABAergic tone and elevated glutamatergic neurotransmission contribute to disrupted excitation/inhibition balance and reduced BZ therapeutic power with benzodiazepine tolerance.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
-
Mus musculus (House mouse)
-
Neuroscience
Preprint on BioRxiv : the Preprint Server for Biology on 1 July 2022 by de Oliveira, L. S., O’Leary, H., et al.
Mutations in the X-linked gene cyclin-dependent kinase-like 5 (CDKL5) cause a severe neurological disorder characterised by early-onset epileptic seizures, autism and intellectual disability (ID). Impaired hippocampal function has been implicated in other models of monogenic forms of autism spectrum disorders and ID and is often linked to epilepsy and behavioural abnormalities. Many individuals with CDKL5 deficiency disorder (CDD) have null mutations and complete loss of CDKL5 protein, therefore in the current study we used a novel Cdkl5 KO rat model to elucidate the impact of CDKL5 loss on cellular excitability and synaptic function of CA1 pyramidal cells (PCs). We hypothesised abnormal pre and/or post synaptic function underlie the enhanced LTP we observe in the hippocampus of Cdkl5 KO rats. We tested this hypothesis using a combination of extracellular and whole-cell electrophysiological recordings, biochemistry, and histology. We show that NMDA receptor function and subunit expression are unaltered throughout development, and Ca 2+ permeable AMPA receptor mediated currents are unchanged in Cdkl5 KO rats. We observe reduced mEPSC frequency accompanied by increased spine density in basal dendrites of CA1 PCs, however we find no evidence supporting an increase in silent synapses when assessed using a minimal stimulation protocol in slices. Additionally, we found no change in paired-pulse ratio, consistent with normal release probability in Cdkl5 KO rats and supported by typical expression of pre-synaptic proteins in synaptosome preparations. Together these data indicate a role for CDKL5 in hippocampal synaptic function and raise the possibility that altered intracellular signalling rather than synaptic deficits might contribute to the altered plasticity.
-
WB
-
Rattus norvegicus (Rat)
In Frontiers in Molecular Neuroscience on 14 December 2021 by Ruden, J. B., Dixit, M., et al.
N-methyl-D-aspartate (NMDA) receptors are critical for higher-order nervous system function, but in previously published protocols to convert human induced pluripotent stem cells (iPSCs) to mature neurons, functional NMDA receptors (NMDARs) are often either not reported or take an extended time to develop. Here, we describe a protocol to convert human iPSC-derived neural progenitor cells (NPCs) to mature neurons in only 37 days. We demonstrate that the mature neurons express functional NMDARs exhibiting ligand-activated calcium flux, and we document the presence of NMDAR-mediated electrically evoked postsynaptic current. In addition to being more rapid than previous procedures, our protocol is straightforward, does not produce organoids which are difficult to image, and does not involve co-culture with rodent astrocytes. This could enhance our ability to study primate/human-specific aspects of NMDAR function and signaling in health and disease.
Copyright © 2021 Ruden, Dixit, Zepeda, Grueter and Dugan.
-
WB
-
Homo sapiens (Human)
-
Neuroscience
-
Stem Cells and Developmental Biology
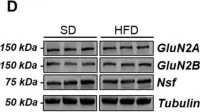
In Int J Mol Sci on 3 April 2021 by Zuliani, I., Lanzillotta, C., et al.
Fig.6.D

-
WB
-
Collected and cropped from Int J Mol Sci by CiteAb, provided under a CC-BY license
Image 1 of 7
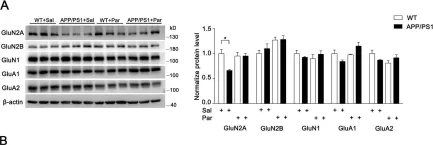
In Transl Neurodegener on 12 May 2020 by Ai, P. H., Chen, S., et al.
Fig.3.A

-
WB
-
Collected and cropped from Transl Neurodegener by CiteAb, provided under a CC-BY license
Image 1 of 7
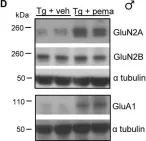
In Life Sci Alliance on 1 April 2019 by Pierrot, N., Ris, L., et al.
Fig.5.D

-
WB
-
Rattus norvegicus (Rat)
Collected and cropped from Life Sci Alliance by CiteAb, provided under a CC-BY license
Image 1 of 7
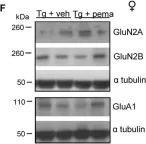
In Life Sci Alliance on 1 April 2019 by Pierrot, N., Ris, L., et al.
Fig.5.F

-
WB
-
Rattus norvegicus (Rat)
Collected and cropped from Life Sci Alliance by CiteAb, provided under a CC-BY license
Image 1 of 7
In Life Sci Alliance on 1 April 2019 by Pierrot, N., Ris, L., et al.
Fig.1.C

-
WB
-
Rattus norvegicus (Rat)
Collected and cropped from Life Sci Alliance by CiteAb, provided under a CC-BY license
Image 1 of 7
In Life Sci Alliance on 1 April 2019 by Pierrot, N., Ris, L., et al.
Fig.1.B

-
WB
-
Rattus norvegicus (Rat)
Collected and cropped from Life Sci Alliance by CiteAb, provided under a CC-BY license
Image 1 of 7
In PLoS One on 25 June 2014 by Fernandes, J., Vieira, M., et al.
Fig.9.A

-
WB
-
Rattus norvegicus (Rat)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 7