Monomeric C-reactive protein (mCRP) is a pro-inflammatory molecule generated by the dissociation of native CRP. Clinical and experimental studies suggest that mCRP deposition in the brain induces Alzheimer's disease (AD) pathology and cognitive loss. Pathological neuroinflammation is increasingly suggested as relevant in AD. Innovative therapies against neuroinflammation are desperately needed, and inhibitors of the enzyme soluble epoxide hydrolase (sEH) are a promising new generation of anti-inflammatory drugs. Mouse primary microglia and BV2 cell line cultures were exposed to mCRP to analyze its pro-inflammatory mechanisms. sEH inhibitors, both newly synthesized UB-SCG-55 and UB-SCG-65, and the reference agent TPPU, were tested for their anti-inflammatory action against mCRP. Phenotypic changes were analyzed through cell imaging techniques, as well as molecular analysis of inflammatory mediators and gene activation pathways. Results show that mCRP triggers a pro-inflammatory response through three main inflammatory pathways: iNOS, NLRP3, and COX-2, followed by increased cytokine generation. Polarization of microglia toward a M1-like phenotype was confirmed by morphological analysis. Also, mCRP can bind to and cross the cell membrane, providing further insight into its mechanisms of action. sEH inhibitors were effective against mCRP induction of a reactive microglial phenotype. The first-line compound UB-SCG-55 emerged as the most potent anti-inflammatory against mCRP injury. Therefore, the direct activation of microglia by mCRP provides evidence of its role in triggering and exacerbating neurodegenerative diseases with a neuroinflammatory component, such as AD. Furthermore, the protection given by inhibitors of sEH confirms its potential as innovative drugs against deleterious effects of neuroinflammation.
Copyright © 2025 The Authors. Published by Elsevier B.V. All rights reserved.
Product Citations: 67
In International Immunopharmacology on 16 May 2025 by Bartra, C., Vuraić, K., et al.
-
WB
-
Immunology and Microbiology
-
Neuroscience
Anti-Inflammatory Effects of Hyeonggaeyeongyo-tang: Evidence from In Vitro and In Vivo Studies.
In Life (Basel, Switzerland) on 2 April 2025 by Lee, K. H., Kim, M. H., et al.
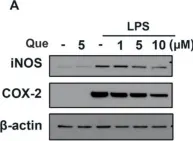
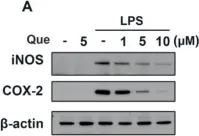
Hyeonggaeyeongyo-tang (HGYGT), a traditional herbal formula, is used to treat inflammatory otorhinolaryngological diseases such as otitis media and sinusitis. In this study, we investigated the anti-inflammatory effects of HGYGT in LPS-stimulated RAW 264.7 cells (in vitro) and a carrageenan (CA)-induced rat paw edema model (in vivo). In LPS-stimulated RAW 264.7 cells, treatment with HGYGT (100 and 300 μg/mL) significantly reduced nitric oxide (NO) production by 24.5% and 51.3%, respectively (p < 0.05, p < 0.01). It also significantly suppressed the production of PGE2 (49.8%), IL-1β (42.7%), IL-6 (45.6%), and TNF-α (47.2%) at 300 μg/mL (p < 0.01). A Western blot analysis confirmed that HGYGT (300 μg/mL) significantly downregulated iNOS and COX-2 expression by 58.4% and 53.1%, respectively, while COX-1 remained unaffected. And HGYGT treatment at 300 μg/mL markedly inhibited NF-κB activation by 44.9% (p < 0.01). Furthermore, HGYGT selectively inhibited JNK phosphorylation by 46.7% (p < 0.01), without significantly affecting ERK1/2 or p38 MAPKs. In the CA-induced rat paw edema model, oral administration of HGYGT (1.0 g/kg) reduced paw swelling by 31.5% at 4 h post-injection (p < 0.01) and significantly decreased iNOS expression in inflamed paw tissues by 43.2% (p < 0.01). A histological analysis revealed that HGYGT (1.0 g/kg) reduced inflammatory cell infiltration by 39.6% in the affected tissue (p < 0.05), demonstrating its anti-inflammatory potential. Our findings demonstrate that HGYGT exerts anti-inflammatory effects by suppressing the JNK and NF-κB signaling pathways in LPS-stimulated RAW 264.7 cells, reducing the production of inflammatory mediators. Notably, HGYGT selectively inhibits COX-2 without affecting COX-1 and preferentially suppresses the JNK pathway. Moreover, its in vivo anti-inflammatory effects were confirmed through iNOS inhibition and histopathological analysis. These findings provide robust scientific evidence supporting the traditional use of HGYGT and its anti-inflammatory properties.
-
Immunology and Microbiology
In Cells on 14 January 2025 by Hong, Y., Ko, G., et al.
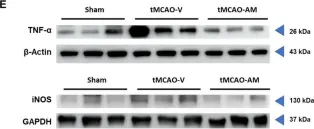
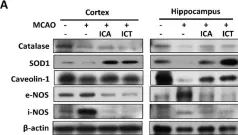
Stroke affects over 12 million people annually, leading to high mortality, long-term disability, and substantial healthcare costs. Although East Asian herbal medicines are widely used for stroke treatment, the pathways of operation they use remain poorly understood. Our study investigates the neuroprotective properties of Astragalus mongholicus (AM) in acute ischemic stroke using photothrombotic (PTB) and transient middle cerebral artery occlusion (tMCAO) mouse models, as well as an in vitro oxygen-glucose deprivation (OGD) model. Post-OGD treatment with AM improved cell viability in mouse neuroblastoma cells, likely by reducing reactive oxygen species (ROS). Mice received short-term (0-2 days) or long-term (0-27 days) AM treatment post-stroke. Infarct size was assessed using a 2,3,5-triphenyl tetrazolium chloride (TTC) staining procedure alongside magnetic resonance imaging (MRI). Neuroprotective metabolites including inositol (Ins), glycerophosphocholine+phosphocholine (GPc+ PCh), N-acetylaspartate+N-acetylaspartylglutamate (NAA+NAAG), creatine + phosphocreatine (Cr+PCr), and glutamine+glutamate (Glx) were analyzed via magnetic resonance spectroscopy (MRS). Gliosis was assessed using GFAP and Iba-1 immunohistochemical markers, while neurological deficits were quantified with modified neurological severity scores (mNSS). Motor and cognitive functions were assessed using cylinder, rotarod, and novel object recognition (NOR) tests. AM treatment significantly reduced ischemic damage and improved neurological outcomes in both acute and chronic stages of PTB and tMCAO models. Additionally, AM increased neuroprotective metabolites levels, reduced gliosis, and decreased oxidative stress, as evidenced by reduced inducible nitric oxide synthase (iNOS). These findings highlight the antioxidant properties of AM and its strong therapeutic potential for promoting recovery after ischemic stroke by alleviating neurological deficits, reducing gliosis, and mitigating oxidative stress.
-
WB
-
Cardiovascular biology
-
Cell Biology
-
Immunology and Microbiology
Brain Injury and Neurodegeneration: Molecular, Functional, and Translational Approach 2.0.
In Biomedicines on 12 November 2024 by Ahluwalia, P., Gaur, P., et al.
In Journal of Neuroinflammation on 23 July 2024 by Kim, D. Y., Kim, S. M., et al.
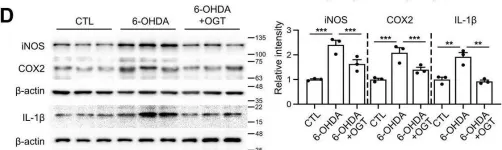
This study investigated the role of O-GlcNAc cycling in Alzheimer's disease-related changes in brain pathophysiology induced by chronic REM sleep deprivation (CSD) in mice. CSD increased amyloid beta (Aβ) and p-Tau accumulation and impaired learning and memory (L/M) function. CSD decreased dendritic length and spine density. CSD also increased the intensity of postsynaptic density protein-95 (PSD-95) staining. All of these Alzheimer's disease (AD) pathogenic changes were effectively reversed through glucosamine (GlcN) treatment by enhancing O-GlcNAcylation. Interestingly, the lelvel of O-GlcNAcylated-Tau (O-Tau) exhibited an opposite trend compared to p-Tau, as it was elevated by CSD and suppressed by GlcN treatment. CSD increased neuroinflammation, as indicated by elevated levels of glial fibrillary acidic protein and IBA-1-positive glial cells in the brain, which were suppressed by GlcN treatment. CSD promoted the phosphorylation of GSK3β and led to an upregulation in the expression of endoplasmic reticulum (ER) stress regulatory proteins and genes. These alterations were effectively suppressed by GlcN treatment. Minocycline not only suppressed neuroinflammation induced by CSD, but it also rescued the decrease in O-GlcNAc levels caused by CSD. Minocycline also reduced AD neuropathy without affecting CSD-induced ER stress. Notably, overexpressing O-GlcNAc transferase in the dentate gyrus region of the mouse brain rescued CSD-induced cognitive dysfunction, neuropathy, neuroinflammation, and ER stress responses. Collectively, our findings reveal that dysregulation of O-GlcNAc cycling underlies CSD-induced AD pathology and demonstrate that restoration of OGlcNAcylation protects against CSD-induced neurodegeneration.
© 2024. The Author(s).
-
Mus musculus (House mouse)
-
Immunology and Microbiology
-
Neuroscience
In Cells on 14 January 2025 by Hong, Y., Ko, G., et al.
Fig.4.E

-
WB
-
Collected and cropped from Cells by CiteAb, provided under a CC-BY license
Image 1 of 17
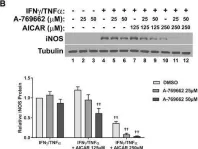
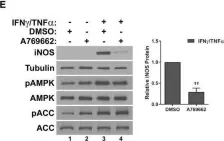
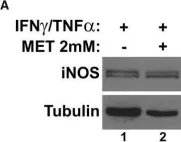
In Cell Death Dis on 23 April 2024 by Kim, D. Y., Kim, S. M., et al.
Fig.5.C

-
WB
-
Mus musculus (House mouse)
Collected and cropped from Cell Death Dis by CiteAb, provided under a CC-BY license
Image 1 of 17
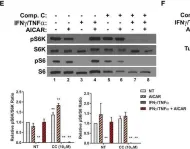
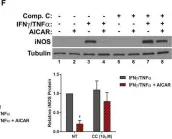
In Cell Death Dis on 23 April 2024 by Kim, D. Y., Kim, S. M., et al.
Fig.7.D

-
WB
-
Mus musculus (House mouse)
Collected and cropped from Cell Death Dis by CiteAb, provided under a CC-BY license
Image 1 of 17
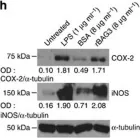
In Nutrients on 19 December 2022 by Yeh, W. L., Huang, B. R., et al.
Fig.5.D

-
WB
-
Mus musculus (House mouse)
Collected and cropped from Nutrients by CiteAb, provided under a CC-BY license
Image 1 of 17
In Nutrients on 24 December 2021 by Tsai, C. F., Chen, G. W., et al.
Fig.2.A

-
WB
-
Mus musculus (House mouse)
Collected and cropped from Nutrients by CiteAb, provided under a CC-BY license
Image 1 of 17
In Nutrients on 24 December 2021 by Tsai, C. F., Chen, G. W., et al.
Fig.3.A

-
WB
-
Mus musculus (House mouse)
Collected and cropped from Nutrients by CiteAb, provided under a CC-BY license
Image 1 of 17
In Biomedicines on 19 November 2021 by Wu, C. T., Chen, M. C., et al.
Fig.5.A

-
WB
-
Collected and cropped from Biomedicines by CiteAb, provided under a CC-BY license
Image 1 of 17
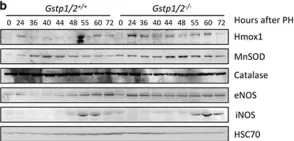
In EMBO Mol Med on 1 July 2018 by Hall, D. T., Griss, T., et al.
Fig.4.D

-
WB
-
Mus musculus (House mouse)
Collected and cropped from EMBO Mol Med by CiteAb, provided under a CC-BY license
Image 1 of 17
In EMBO Mol Med on 1 July 2018 by Hall, D. T., Griss, T., et al.
Fig.7.B

-
WB
-
Mus musculus (House mouse)
Collected and cropped from EMBO Mol Med by CiteAb, provided under a CC-BY license
Image 1 of 17
In EMBO Mol Med on 1 July 2018 by Hall, D. T., Griss, T., et al.
Fig.6.E

-
WB
-
Mus musculus (House mouse)
Collected and cropped from EMBO Mol Med by CiteAb, provided under a CC-BY license
Image 1 of 17
In EMBO Mol Med on 1 July 2018 by Hall, D. T., Griss, T., et al.
Fig.5.E

-
WB
-
Mus musculus (House mouse)
Collected and cropped from EMBO Mol Med by CiteAb, provided under a CC-BY license
Image 1 of 17
In EMBO Mol Med on 1 July 2018 by Hall, D. T., Griss, T., et al.
Fig.5.F

-
WB
-
Mus musculus (House mouse)
Collected and cropped from EMBO Mol Med by CiteAb, provided under a CC-BY license
Image 1 of 17
In EMBO Mol Med on 1 July 2018 by Hall, D. T., Griss, T., et al.
Fig.2.A

-
WB
-
Mus musculus (House mouse)
Collected and cropped from EMBO Mol Med by CiteAb, provided under a CC-BY license
Image 1 of 17
In Nat Commun on 2 November 2015 by Rosati, A., Basile, A., et al.
Fig.1.H

-
WB
-
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 17
In Nat Commun on 2 November 2015 by Rosati, A., Basile, A., et al.
Fig.2.C

-
WB
-
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 17
In Cell Death Dis on 15 January 2015 by Pajaud, J., Ribault, C., et al.
Fig.7.B

-
WB
-
Mus musculus (House mouse)
Collected and cropped from Cell Death Dis by CiteAb, provided under a CC-BY license
Image 1 of 17
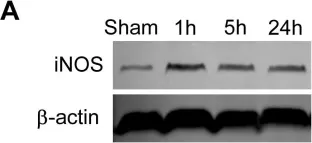
In PLoS One on 22 February 2012 by Wei, D., Ren, C., et al.
Fig.7.A

-
WB
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 17