Most wounds form scars without hair follicles. However, in the wound-induced hair neogenesis (WIHN) model of skin regeneration, wounds regenerate hair follicles if tissue rigidity is optimal. Although WIHN depends on Wnt signaling, whether Wnt performs a mechanoregulatory role that contributes to regeneration remains uncharacterized. Here, we demonstrate that Wnt signaling affects mechanosensitivity at both cellular and tissue levels to drive WIHN. Atomic force microscopy revealed an attenuated substrate rigidity response in epidermal but not dermal cells of healing wounds. Super-resolution microscopy and nanoneedle probing of intracellular compartments in live human keratinocytes revealed that Wnt-induced chromatin remodeling triggers a 10-fold drop in nuclear rigidity without jeopardizing the nucleocytoskeletal mechanical coupling. Mechanistically, Wnt signaling orchestrated a massive reorganization of actin architecture and recruited adherens junctions to generate a mechanical syncytium-a cohesive contractile unit with superior capacity for force coordination and collective durotaxis. Collectively, our findings unveil Wnt signaling's mechanoregulatory role that manipulates the machinery of mechanotransduction to drive regeneration.
Product Citations: 8
Wnt signaling modulates mechanotransduction in the epidermis to drive hair follicle regeneration.
In Science Advances on 21 February 2025 by Oak, A. S. W., Bagchi, A., et al.
In Respiratory Research on 10 June 2023 by Hernandez-Lara, M. A., Yadav, S. K., et al.
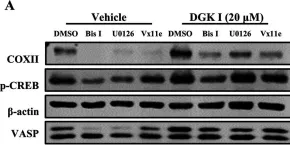
Diacylglycerol kinase (DGK) regulates intracellular signaling and functions by converting diacylglycerol (DAG) into phosphatidic acid. We previously demonstrated that DGK inhibition attenuates airway smooth muscle (ASM) cell proliferation, however, the mechanisms mediating this effect are not well established. Given the capacity of protein kinase A (PKA) to effect inhibition of ASM cells growth in response to mitogens, we employed multiple molecular and pharmacological approaches to examine the putative role of PKA in the inhibition of mitogen-induced ASM cell proliferation by the small molecular DGK inhibitor I (DGK I).
We assayed cell proliferation using CyQUANT™ NF assay, protein expression and phosphorylation using immunoblotting, and prostaglandin E2 (PGE2) secretion by ELISA. ASM cells stably expressing GFP or PKI-GFP (PKA inhibitory peptide-GFP chimera) were stimulated with platelet-derived growth factor (PDGF), or PDGF + DGK I, and cell proliferation was assessed.
DGK inhibition reduced ASM cell proliferation in cells expressing GFP, but not in cells expressing PKI-GFP. DGK inhibition increased cyclooxygenase II (COXII) expression and PGE2 secretion over time to promote PKA activation as demonstrated by increased phosphorylation of (PKA substrates) VASP and CREB. COXII expression and PKA activation were significantly decreased in cells pre-treated with pan-PKC (Bis I), MEK (U0126), or ERK2 (Vx11e) inhibitors suggesting a role for PKC and ERK in the COXII-PGE2-mediated activation of PKA signaling by DGK inhibition.
Our study provides insight into the molecular pathway (DAG-PKC/ERK-COXII-PGE2-PKA) regulated by DGK in ASM cells and identifies DGK as a potential therapeutic target for mitigating ASM cell proliferation that contributes to airway remodeling in asthma.
© 2023. The Author(s).
-
WB
-
Homo sapiens (Human)
Autocrine regulation of airway smooth muscle contraction by diacylglycerol kinase.
In Journal of Cellular Physiology on 1 January 2022 by Yadav, S. K., Sharma, P., et al.
Diacylglycerol kinase (DGK), a lipid kinase, catalyzes the conversion of diacylglycerol (DAG) to phosphatidic acid, thereby terminating DAG-mediated signaling by Gq-coupled receptors that regulate contraction of airway smooth muscle (ASM). A previous study from our laboratory demonstrated that DGK inhibition or genetic ablation leads to reduced ASM contraction and provides protection for allergen-induced airway hyperresponsiveness. However, the mechanism by which DGK regulates contractile signaling in ASM is not well established. Herein, we investigated the role of prorelaxant cAMP-protein kinase A (PKA) signaling in DGK-mediated regulation of ASM contraction. Pretreatment of human ASM cells with DGK inhibitor I activated PKA as demonstrated by the phosphorylation of PKA substrates, VASP, Hsp20, and CREB, which was abrogated when PKA was inhibited pharmacologically or molecularly using overexpression of the PKA inhibitor peptide, PKI. Furthermore, inhibition of DGK resulted in induction of cyclooxygenase (COX) and generation of prostaglandin E2 (PGE2 ) with concomitant activation of Gs-cAMP-PKA signaling in ASM cells in an autocrine/paracrine fashion. Inhibition of protein kinase C (PKC) or extracellular-signal-regulated kinase (ERK) attenuated DGK-mediated production of PGE2 and activation of cAMP-PKA signaling in human ASM cells, suggesting that inhibition of DGK activates the COX-PGE2 pathway in a PKC-ERK-dependent manner. Finally, DGK inhibition-mediated attenuation of contractile agonist-induced phosphorylation of myosin light chain 20 (MLC-20), a marker of ASM contraction, involves COX-mediated cAMP production and PKA activation in ASM cells. Collectively these findings establish a novel mechanism by which DGK regulates ASM contraction and further advances DGK as a potential therapeutic target to provide effective bronchoprotection in asthma.
© 2021 Wiley Periodicals LLC.
-
Homo sapiens (Human)
-
Endocrinology and Physiology
A primer for measuring cGMP signaling and cGMP-mediated vascular relaxation.
In Nitric Oxide : Biology and Chemistry / Official Journal of the Nitric Oxide Society on 1 December 2021 by Straub, A. C. & Beuve, A.
Soluble guanylyl cyclase (sGC, also called GC1) is the main receptor for nitric oxide (NO) that catalyzes the production of the second messenger molecule, 3'5' cyclic guanosine monophosphate (cGMP) leading to vasorelaxation, and inhibition of leukocyte recruitment and platelet aggregation. Enhancing cGMP levels, through sGC agonism or inhibition of cGMP breakdown via phosphodiesterase inhibition, has yielded FDA approval for several cGMP modifier therapies for treatment of cardiovascular and pulmonary diseases. While basic research continues to improve our understanding of cGMP signaling and as new therapies evolve to elevate cGMP levels, we provide a short methodological primer for measuring cGMP and cGMP-mediated vascular relaxation for investigators.
Copyright © 2021. Published by Elsevier Inc.
The HIF target ATG9A is essential for epithelial barrier function and tight junction biogenesis.
In Molecular Biology of the Cell on 15 September 2020 by Dowdell, A. S., Cartwright, I. M., et al.
Intestinal epithelial cells (IECs) exist in a metabolic state of low oxygen tension termed "physiologic hypoxia." An important factor in maintaining intestinal homeostasis is the transcription factor hypoxia-inducible factor (HIF), which is stabilized under hypoxic conditions and mediates IEC homeostatic responses to low oxygen tension. To identify HIF transcriptional targets in IEC, chromatin immunoprecipitation (ChIP) was performed in Caco-2 IECs using HIF-1α- or HIF-2α-specific antibodies. ChIP-enriched DNA was hybridized to a custom promoter microarray (termed ChIP-chip). This unbiased approach identified autophagy as a major HIF-1-targeted pathway in IEC. Binding of HIF-1 to the ATG9A promoter, the only transmembrane component within the autophagy pathway, was particularly enriched by exposure of IEC to hypoxia. Validation of this ChIP-chip revealed prominent induction of ATG9A, and luciferase promoter assays identified a functional hypoxia response element upstream of the TSS. Hypoxia-mediated induction of ATG9A was lost in cells lacking HIF-1. Strikingly, we found that lentiviral-mediated knockdown (KD) of ATG9A in IECs prevents epithelial barrier formation by >95% and results in significant mislocalization of multiple tight junction (TJ) proteins. Extensions of these findings showed that ATG9A KD cells have intrinsic abnormalities in the actin cytoskeleton, including mislocalization of the TJ binding protein vasodilator-stimulated phosphoprotein. These results implicate ATG9A as essential for multiple steps of epithelial TJ biogenesis and actin cytoskeletal regulation. Our findings have novel applicability for disorders that involve a compromised epithelial barrier and suggest that targeting ATG9A may be a rational strategy for future therapeutic intervention.
-
ICC-IF
-
Cell Biology
In Respir Res on 10 June 2023 by Hernandez-Lara, M. A., Yadav, S. K., et al.
Fig.3.A

-
WB
-
Homo sapiens (Human)
Collected and cropped from Respir Res by CiteAb, provided under a CC-BY license
Image 1 of 1