Birnaviruses are a group of double-stranded RNA (dsRNA) viruses infecting birds, fish, and insects. Early endosomes (EE) constitute the platform for viral replication. Here, we study the mechanism of birnaviral targeting of EE membranes. Using the Infectious Bursal Disease Virus (IBDV) as a model, we validate that the viral protein 3 (VP3) binds to phosphatidylinositol-3-phosphate (PI3P) present in EE membranes. We identify the domain of VP3 involved in PI3P-binding, named P2 and localized in the core of VP3, and establish the critical role of the arginine at position 200 (R200), conserved among all known birnaviruses. Mutating R200 abolishes viral replication. Moreover, we propose a two-stage modular mechanism for VP3 association with EE. Firstly, the carboxy-terminal region of VP3 adsorbs on the membrane, and then the VP3 core reinforces the membrane engagement by specifically binding PI3P through its P2 domain, additionally promoting PI3P accumulation.
© 2024, Zanetti, Fernandez et al.
Product Citations: 327
On the role of VP3-PI3P interaction in birnavirus endosomal membrane targeting.
In eLife on 6 March 2025 by Zanetti, F. A., Fernandez, I., et al.
-
WB
-
Cell Biology
In Frontiers in Cellular Neuroscience on 7 February 2025 by Speca, D. J., He, C. W., et al.
The transmembrane protein Synapse Differentiation Induced Gene 4 (SynDIG4), also known as Proline-rich transmembrane protein 1 (PRRT1), is an AMPA-type glutamate receptor (AMPAR) auxiliary factor that is necessary for maintaining extra-synaptic pools of GluA1. Loss of SynDIG4, and the subsequent decrease in extra-synaptic GluA1, has been found to significantly impact synaptic plasticity in the hippocampus. However, how SynDIG4 establishes and maintains these pools is unclear. Previous studies suggested that endocytic machinery is important for maintaining a pool of mobile surface AMPARs, and that proteins associated with such cellular machinery are critical for proper protein trafficking and internalization. Given that SynDIG4 co-localizes with GluA1 in early and recycling endosomes in cultured hippocampal neurons, we sought to identify the sorting signals that target SynDIG4 to endosomes to further elucidate the role of SynDIG4 in GluA1 trafficking. In this study, we report that SynDIG4 possesses a YxxΦ sorting motif, 178-YVPV-181, responsible for binding to the AP-2 complex cargo-sorting subunit μ2. This motif appears critical for proper SynDIG4 internalization, as SynDIG4 mutant 178-AVPA-181, which disrupts binding to μ2, induces aberrant SynDIG4 accumulation at the plasma-membrane of heterologous cells and primary rat hippocampal neurons. We also show that SynDIG4 mutants lacking an endocytic signal co-localize with GluA1 but less so with GluA2 on the surface of heterologous cells. Furthermore, we show that another family member, SynDIG1, is enriched in the trans-Golgi network (TGN) and can traffic between the TGN and plasma membrane. We have identified a non-canonical μ2 binding sequence in SynDIG1 that induces aberrant accumulation at the plasma membrane of heterologous cells and primary rat hippocampal neurons, suggesting a conserved role for μ2-mediated endocytosis within the SynDIG family. These results provide important insight into the mechanisms by which SynDIG proteins are targeted to endosomal compartments as a step in understanding SynDIG-mediated regulation of AMPAR trafficking.
Copyright © 2025 Speca, He, Meyer, Scott and Díaz.
-
ICC-IF
-
Chlorocebus aethiops (Grivet monkey)
-
Neuroscience
In Cell Death Discovery on 1 February 2025 by Xu, L., Guo, J., et al.
Small GTPases play a critical role as regulatory molecules in signaling transduction and various cellular processes, contributing to the development of human diseases, including cancers. GPN3, an evolutionarily conserved member of the GPN-loop GTPase subfamily classified in 2007 according to its structure, has limited knowledge regarding its cellular functions and molecular mechanisms. In this study, we demonstrate that GPN3 interacts with clathrin light chain A (CLTA), a vesicle coat protein, as well as clathrin-mediated endocytosis associated modulators AP2B1 and AP2S1. Upregulation of GPN3 leads to the inhibition of clathrin-coated pit invagination. Furthermore, we discovered that GPN3 interacts with the epidermal growth factor receptor (EGFR) and regulates the co-localization of EGFR and CLTA, as well as the localization of EGFR in early endosomes upon EGF stimulation. As a result, this leads to a decrease in endocytic levels of EGFR and an increase in the accumulation of EGFR on the cell membrane surface, thereby prolonging activation of EGFR signaling. The functional effects exerted by GPN3 are dependent on cellular levels of GTP abundance. Furthermore, our findings indicate that aberrant overexpression of GPN3 is observed in non-small cell lung cancer (NSCLC) tissues compared to adjacent normal tissues, and high expression levels of GPN3 are associated with poor prognosis for NSCLC patients. Collectively, these findings reveal that GPN3 acts as an oncogene promoting cell proliferation and migration in NSCLC through regulation of clathrin-dependent EGFR endocytosis. These results suggest that targeting GPN3 could serve as a novel prognostic biomarker and therapeutic strategy for NSCLC treatment.
© 2025. The Author(s).
-
ICC-IF
-
Homo sapiens (Human)
-
Cancer Research
In Scientific Reports on 8 January 2025 by Bhat, M. A., Hleihil, M., et al.
GABAB receptors mediate prolonged inhibition in the brain and are important for keeping neuronal excitation and inhibition in a healthy balance. However, under excitotoxic/ischemic conditions, GABAB receptors are downregulated by dysregulated endocytic trafficking and can no longer counteract the severely enhanced excitation, eventually triggering neuronal death. Recently, we developed interfering peptides targeting protein-protein interactions involved in downregulating the receptors. Treatment with these peptides restored GABAB receptor expression after an ischemic insult and thereby inhibited neuronal overexcitation and progressive neuronal death. In this study, we searched for GABAB receptor interactions that specifically occur under ischemic conditions. We found that the E3 ubiquitin ligase MARCH1 is specifically upregulated under ischemic/excitotoxic conditions. Upregulated MARCH1 interacts with GABAB receptors and triggered downregulation of plasma membrane GABAB receptors by inhibiting fast recycling of the receptors. We developed an interfering peptide that inhibits the MARCH1/GABAB receptor interaction. Treatment of cultured neurons subjected to ischemic stress with this peptide restored receptor expression and as a consequence stopped progressive neuronal death. Thus, inhibiting the interaction of GABAB receptors with MARCH1 to restore cell surface receptor expression might be a promising strategy to prevent progressive neuronal death induced by ischemic conditions.
© 2025. The Author(s).
Spatiotemporal recruitment of the ubiquitin-specific protease USP8 directs endosome maturation.
In eLife on 22 November 2024 by Miao, Y., Du, Y., et al.
The spatiotemporal transition of small GTPase Rab5 to Rab7 is crucial for early-to-late endosome maturation, yet the precise mechanism governing Rab5-to-Rab7 switching remains elusive. USP8, a ubiquitin-specific protease, plays a prominent role in the endosomal sorting of a wide range of transmembrane receptors and is a promising target in cancer therapy. Here, we identified that USP8 is recruited to Rab5-positive carriers by Rabex5, a guanine nucleotide exchange factor (GEF) for Rab5. The recruitment of USP8 dissociates Rabex5 from early endosomes (EEs) and meanwhile promotes the recruitment of the Rab7 GEF SAND-1/Mon1. In USP8-deficient cells, the level of active Rab5 is increased, while the Rab7 signal is decreased. As a result, enlarged EEs with abundant intraluminal vesicles accumulate and digestive lysosomes are rudimentary. Together, our results reveal an important and unexpected role of a deubiquitinating enzyme in endosome maturation.
© 2024, Miao, Du et al.
-
Cell Biology
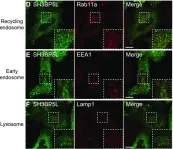
In Life Sci Alliance on 1 March 2023 by Da Graca, J., Charles, J., et al.
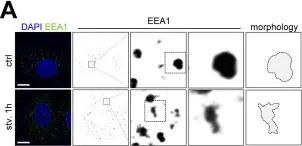
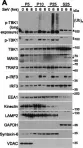
Fig.1.A

-
ICC-IF
-
Homo sapiens (Human)
Collected and cropped from Life Sci Alliance by CiteAb, provided under a CC-BY license
Image 1 of 13
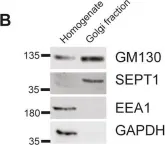
In Elife on 26 August 2020 by Liu, Y., Li, L., et al.
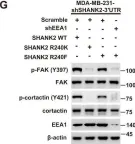
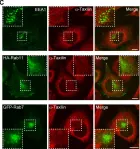
Fig.6.G

-
WB
-
Collected and cropped from Elife by CiteAb, provided under a CC-BY license
Image 1 of 13
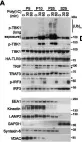
In Elife on 26 August 2020 by Liu, Y., Li, L., et al.
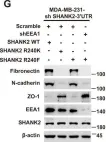
Fig.7.G

-
WB
-
Collected and cropped from Elife by CiteAb, provided under a CC-BY license
Image 1 of 13
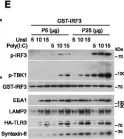
In Life Sci Alliance on 1 April 2019 by Goto-Ito, S., Morooka, N., et al.
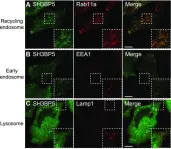
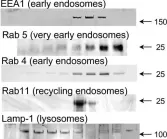
Fig.5.A

-
ICC-IF
-
Homo sapiens (Human)
Collected and cropped from Life Sci Alliance by CiteAb, provided under a CC-BY license
Image 1 of 13
In Life Sci Alliance on 1 April 2019 by Goto-Ito, S., Morooka, N., et al.
Fig.5.D

-
ICC-IF
-
Homo sapiens (Human)
Collected and cropped from Life Sci Alliance by CiteAb, provided under a CC-BY license
Image 1 of 13
In J Cell Sci on 1 February 2019 by Song, K., Gras, C., et al.
Fig.1.B

-
WB
-
Homo sapiens (Human)
Collected and cropped from J Cell Sci by CiteAb, provided under a CC-BY license
Image 1 of 13
In BMC Biol on 18 August 2016 by Pourcelot, M., Zemirli, N., et al.
Fig.2.A

-
WB
-
Homo sapiens (Human)
Collected and cropped from BMC Biol by CiteAb, provided under a CC-BY license
Image 1 of 13
In BMC Biol on 18 August 2016 by Pourcelot, M., Zemirli, N., et al.
Fig.2.E

-
WB
-
Homo sapiens (Human)
Collected and cropped from BMC Biol by CiteAb, provided under a CC-BY license
Image 1 of 13
In BMC Biol on 18 August 2016 by Pourcelot, M., Zemirli, N., et al.
Fig.1.A

-
WB
-
Homo sapiens (Human)
Collected and cropped from BMC Biol by CiteAb, provided under a CC-BY license
Image 1 of 13
In PLoS One on 3 April 2014 by Sakane, H., Horii, Y., et al.
Fig.4.C

-
ICC-IF
-
Homo sapiens (Human)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 13
In PLoS One on 23 August 2011 by Scharadin, T. M., Jiang, H., et al.
Fig.7.A

-
WB
-
Homo sapiens (Human)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 13
In Mol Pain on 10 March 2009 by Garzón, J., de la Torre-Madrid, E., et al.
Fig.3.C

-
WB
-
Collected and cropped from Mol Pain by CiteAb, provided under a CC-BY license
Image 1 of 13
In Mol Pain on 17 July 2007 by Rodríguez-Muñoz, M., de la Torre-Madrid, E., et al.
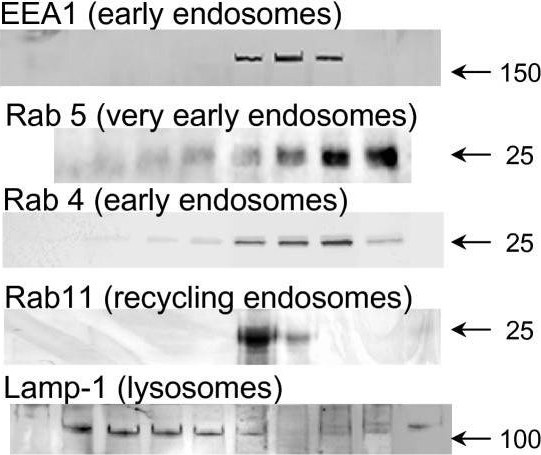
Fig.4.A
-
WB
-
Mus musculus (House mouse)
Collected and cropped from Mol Pain by CiteAb, provided under a CC-BY license
Image 1 of 13