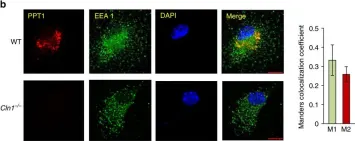
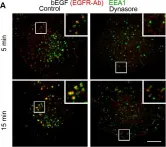
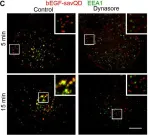
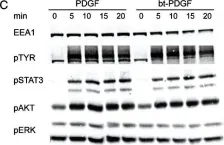
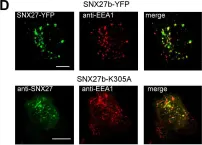
Three-dimensional cell models, such as spheroids, represent a more physiological arrangement in which cells can grow, allowing them to develop cell-cell interactions in all dimensions. The most common methods for growing spheroids are scaffold-based, typically using either extracellular matrix or hydrogels as a physical support for the cellular assembly. One key problem with this approach is that the spheroids that are produced can be highly variable in size and shape. The protocol presented here allows for the systematic production of uniform spheroids in a short time frame by utilising a micropatterned plate. We show that spheroids can be used to investigate fundamental research questions, such as how the endomembrane system is organised in cells. Our protocol can be used in a manual or automated manner, potentially allowing scaling up for screening applications. Furthermore, without the complication of removing the spheroids from the extracellular matrix or hydrogel, as would be required in scaffold-based systems, spheroids can easily be used in other downstream applications. Key features • This work builds upon the method developed by Monjaret et al. [1] and establishes a robust method to produce spheroids from HeLa Kyoto cells. • This protocol generates consistent populations of spheroids that can be used to investigate organelle biology and membrane trafficking pathways. • Entire spheroids can be analysed in a volumetric manner. • This method can be used in both manual and automated pipelines, thereby facilitating use in high-throughput and high-content screens.
©Copyright : © 2025 The Authors; This is an open access article under the CC BY license.
Product Citations: 452
In Bio-protocol on 5 June 2025 by Mysior, M. M. & Simpson, J. C.
SNX10 functions as a modulator of piecemeal mitophagy and mitochondrial bioenergetics.
In The Journal of Cell Biology on 5 May 2025 by Trachsel-Moncho, L., Veroni, C., et al.
We here identify the endosomal protein SNX10 as a negative regulator of piecemeal mitophagy of OXPHOS machinery components. In control conditions, SNX10 localizes to early endocytic compartments in a PtdIns3P-dependent manner and modulates endosomal trafficking but also shows dynamic connections with mitochondria. Upon hypoxia-mimicking conditions, SNX10 localizes to late endosomal structures containing selected mitochondrial proteins, including COX-IV and SAMM50, and the autophagy proteins SQSTM1/p62 and LC3B. The turnover of COX-IV was enhanced in SNX10-depleted cells, with a corresponding reduced mitochondrial respiration and citrate synthase activity. Importantly, zebrafish larvae lacking Snx10 show reduced levels of Cox-IV, as well as elevated ROS levels and ROS-mediated cell death in the brain, demonstrating the in vivo relevance of SNX10-mediated modulation of mitochondrial bioenergetics.
© 2025 Trachsel-Moncho et al.
-
Cell Biology
Preprint on BioRxiv : the Preprint Server for Biology on 14 April 2025 by Gao, J., Cong, C., et al.
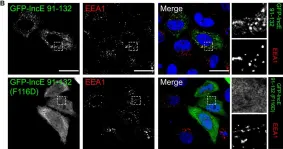
ABSTRACT Many transmembrane (TM) receptors undergo essential post-translational modification in the Golgi prior to their delivery to the plasma membrane. Whether and how the passage and accompanied modification of these receptors across the Golgi is controlled remains unclear. Here, we show that leptin receptor overlapping transcript (LEPROT) and LEPROT-like 1 (LEPROTL1) regulate receptor activation by securing their sufficient Golgi retention. LEPROTs localize to cis and medial Golgi in a COPI-dependent manner. Deletion of LEPROTs in cells causes expedited release of receptors transiting through the Golgi, and leakage of some Golgi-enriched proteins into the endomembrane compartments. LEPROTs interact directly with COPI coats and simultaneously engage a variety of integral membrane proteins with relatively long TM domains at acidic pH. Loss of LEPROTs dysregulates receptor activity, including that of EGFR and TFRC, due to defective modification. Collectively, LEPROTs serve as a class of COPI adaptors for TM receptors, ensuring adequate preparation, which is vital for subsequent action on the plasma membrane.
-
Cell Biology
Astrocytic polyamine transport by ATP13A4 tunes excitatory synaptic transmission
Preprint on MedRxiv : the Preprint Server for Health Sciences on 6 April 2025 by van Veen, S., Irala, D., et al.
Polyamines, such as spermidine and spermine, are essential for brain function and neurodevelopment. These soluble molecules modulate glial and neuronal ion channels, transporters, and receptors, contributing to cellular communication in the brain. Within the brain, polyamines primarily accumulate in astrocytes, but the mechanisms of polyamine uptake in astrocytes and the physiological relevance of this process in brain function remain poorly understood. Here, we identified ATP13A4, a P5B-type transport ATPase predominantly expressed in astrocytes, as a key polyamine transporter that regulates polyamine uptake and homeostasis in astrocytes. Using primary cultures and rodent models, we show that ATP13A4 deficiency reduces astrocyte morphological complexity and increases excitatory synapse formation. Exogenous spermidine application recapitulated these effects, suggesting that astrocytes play a critical role in clearing extracellular polyamines. Moreover, we identified a novel homozygous p.E276K variant of ATP13A4 in a patient with intellectual disability and a heterozygous deletion spanning exons 19-25 in a patient with epilepsy. Additionally, we characterized two ATP13A4 variants previously associated with autism and language impairment. These variants exhibited loss-of-function phenotypes, pointing to a link between imbalanced polyamine homeostasis and neurodevelopmental disorders. Correspondingly, Atp13a4 KO mice exhibit mild, sex-specific behavioral deficits. Female KO mice display subtle changes in anxiety-like behavior, spatial learning, motor coordination, and seizure susceptibility, aligning with features observed in patients with loss-of-function ATP13A4 mutations. In summary, astrocytes take up extracellular polyamines via ATP13A4, which regulate astrocyte arborization and excitatory synapse formation, significantly impacting neurodevelopment and behavior. This work provides the first direct link between dysfunctional astrocytic polyamine transport and perturbations in brain development, providing novel insights into the molecular mechanisms underlying neurodevelopmental disorders.
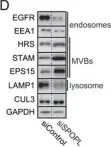
KIF20A cooperates with Myosin II to drive endosomal carriers fission and protein trafficking
Preprint on BioRxiv : the Preprint Server for Biology on 2 April 2025 by Selot, J., Lasbareilles, S., et al.
ABSTRACT Formation of membrane transport carrier relies on cytoskeleton coordination, however how microtubules and actin dynamics cooperate is still poorly understood. In this study, we reveal a novel mechanism by which a kinesin, KIF20A, regulates actomyosin and branched actin dynamics in coordination with Myosin II, facilitating the fission of transport intermediates at early endosomes. We demonstrate that KIF20A is required for maintaining endosomal homeostasis as its inhibition results in the enlargement of both early and late endosomes. Moreover, our findings demonstrate that KIF20A is required for the proper trafficking of Transferrin (Tf), and for ensuring the correct plasma membrane localization of β1 Integrin, a key protein that is critical for cell adhesion and migration. Collectively, these results highlight a new pivotal role of KIF20A as a key regulator of endosomal dynamics and function, contributing to metastatic potential.
-
Cell Biology
In Front Cell Dev Biol on 3 July 2023 by Patil, S. S., Panchal, V., et al.
Fig.1.A

-
WB
-
Collected and cropped from Front Cell Dev Biol by CiteAb, provided under a CC-BY license
Image 1 of 24
In Front Cell Dev Biol on 3 July 2023 by Patil, S. S., Panchal, V., et al.
Fig.1.B

-
WB
-
Collected and cropped from Front Cell Dev Biol by CiteAb, provided under a CC-BY license
Image 1 of 24
In Int J Mol Sci on 30 November 2021 by Kim, J. H., Choi, H. S., et al.
Fig.2.A

-
ICC-IF
-
Collected and cropped from Int J Mol Sci by CiteAb, provided under a CC-BY license
Image 1 of 24
In EMBO J on 17 May 2021 by Breyer, F., Härtlova, A., et al.
Fig.5.H

-
ICC-IF
-
Collected and cropped from EMBO J by CiteAb, provided under a CC-BY license
Image 1 of 24
In Commun Biol on 26 March 2021 by Batlle, C., Calvo, I., et al.
Fig.7.A

-
WB
-
Collected and cropped from Commun Biol by CiteAb, provided under a CC-BY license
Image 1 of 24
In Elife on 23 December 2019 by Harde, E., Nicholson, L., et al.
Fig.5.A

-
ICC-IF
-
Collected and cropped from Elife by CiteAb, provided under a CC-BY license
Image 1 of 24
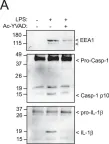
In Sci Rep on 8 April 2019 by Baroja-Mazo, A., Compan, V., et al.
Fig.3.A

-
WB
-
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 24
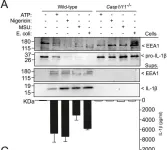
In Sci Rep on 8 April 2019 by Baroja-Mazo, A., Compan, V., et al.
Fig.2.A

-
WB
-
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 24
In Mol Cell Biochem on 1 November 2018 by Radif, Y., Ndiaye, H., et al.
Fig.2.A

-
WB
-
Homo sapiens (Human)
Collected and cropped from Mol Cell Biochem by CiteAb, provided under a CC-BY license
Image 1 of 24
In Elife on 22 February 2018 by Hsu, F., Spannl, S., et al.
Fig.9.D

-
WB
-
Collected and cropped from Elife by CiteAb, provided under a CC-BY license
Image 1 of 24
In Elife on 22 February 2018 by Hsu, F., Spannl, S., et al.
Fig.6.A

-
WB
-
Collected and cropped from Elife by CiteAb, provided under a CC-BY license
Image 1 of 24
In Front Mol Neurosci on 30 September 2017 by Martín-Maestro, P., Gargini, R., et al.
Fig.2.A

-
ICC-IF
-
Homo sapiens (Human)
Collected and cropped from Front Mol Neurosci by CiteAb, provided under a CC-BY license
Image 1 of 24
In Nat Commun on 7 March 2017 by Bagh, M. B., Peng, S., et al.
Fig.4.B

-
ICC-IF
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 24
In Elife on 22 February 2017 by Paul, B., Kim, H. S., et al.
Fig.6.B

-
ICC-IF
-
Homo sapiens (Human)
Collected and cropped from Elife by CiteAb, provided under a CC-BY license
Image 1 of 24
In PLoS One on 28 June 2016 by Chaubey, P. M., Hofstetter, L., et al.
Fig.6.B

-
IHC-IF
-
Rattus norvegicus (Rat)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 24
In Elife on 23 March 2016 by Gschweitl, M., Ulbricht, A., et al.
Fig.2.D

-
WB
-
Homo sapiens (Human)
Collected and cropped from Elife by CiteAb, provided under a CC-BY license
Image 1 of 24
In Oncotarget on 2 February 2016 by Salova, A. V., Belyaeva, T. N., et al.
Fig.7.A

-
ICC-IF
-
Collected and cropped from Oncotarget by CiteAb, provided under a CC-BY license
Image 1 of 24
In Oncotarget on 2 February 2016 by Salova, A. V., Belyaeva, T. N., et al.
Fig.7.C

-
ICC-IF
-
Collected and cropped from Oncotarget by CiteAb, provided under a CC-BY license
Image 1 of 24
In Traffic on 1 June 2013 by Sadowski, Ł., Jastrzębski, K., et al.
Fig.1.C

-
WB
-
Collected and cropped from Traffic by CiteAb, provided under a CC-BY license
Image 1 of 24
In PLoS One on 29 March 2013 by Balana, B., Bahima, L., et al.
Fig.3.D

-
ICC-IF
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 24
In PLoS Pathog on 13 April 2012 by Gendrin, C., Contreras-Martel, C., et al.
Fig.5.A

-
ICC-IF
-
Collected and cropped from PLoS Pathog by CiteAb, provided under a CC-BY license
Image 1 of 24
In PLoS Pathog on 13 April 2012 by Gendrin, C., Contreras-Martel, C., et al.
Fig.5.B

-
ICC-IF
-
Collected and cropped from PLoS Pathog by CiteAb, provided under a CC-BY license
Image 1 of 24
In PLoS Pathog on 29 July 2010 by Jambou, R., Combes, V., et al.
Fig.4.A,B

-
ICC-IF
-
Homo sapiens (Human)
Collected and cropped from PLoS Pathog by CiteAb, provided under a CC-BY license
Image 1 of 24
In PLoS One on 31 May 2007 by Ignoul, S., Simaels, J., et al.
Fig.4.A

-
ICC-IF
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 24