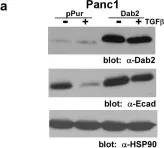
Pancreatic ductal adenocarcinoma (PDAC) is a highly aggressive cancer type characterized by rapid metastasis and resistance to chemotherapy, properties that are shared by cancer stem cells (CSCs). In pancreatic cancer, tumor cells which possess the properties of CSCs also phenotypically resemble cells that have undergone epithelial-to-mesenchymal transition or EMT. Disabled-2 (Dab2) is a multifunctional scaffold protein frequently downregulated in cancer that has been linked to the process of EMT. However, the role of Dab2 in pancreatic cancer development and progression remains unclear. Downregulation of Dab2 expression in pancreatic cancer cell lines was found to trigger induction of genes characteristic of EMT and the CSC phenotype, while overexpression of Dab2 in the Panc1 cell line blocked the process of TGFβ-stimulated EMT. In addition, selective inhibition of the TGFβRI/RII receptors was found to reverse genes altered by Dab2 downregulation. Dab2 mRNA expression was found to be decreased in PDAC tumor samples, as compared to levels observed in normal pancreatic tissue. Methylation of the Dab2 gene promoter was demonstrated in Stage I PDAC tumors and in the MiaPaCa2 cell line, suggesting that promoter methylation may silence Dab2 expression early in pancreatic cancer progression. These results suggest that Dab2 may function as a tumor suppressor in pancreatic cancer by modulation of the TGFβ-stimulated EMT and CSC phenotype.
Product Citations: 17
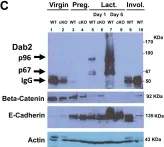
Loss of Disabled-2 Expression in Pancreatic Cancer Progression.
In Scientific Reports on 17 May 2019 by Hocevar, B. A.
-
WB
-
Homo sapiens (Human)
-
Cancer Research
GATA6 phosphorylation by Erk1/2 propels exit from pluripotency and commitment to primitive endoderm.
In Developmental Biology on 1 April 2018 by Meng, Y., Moore, R., et al.
The transcription factor GATA6 and the Fgf/Ras/MAPK signaling pathway are essential for the development of the primitive endoderm (PrE), one of the two lineages derived from the pluripotent inner cell mass (ICM) of mammalian blastocysts. A mutant mouse line in which Gata6-coding exons are replaced with H2BGFP (histone H2B Green Fluorescence Protein fusion protein) was developed to monitor Gata6 promoter activity. In the Gata6-H2BGFP heterozygous blastocysts, the ICM cells that initially had uniform GFP fluorescence signal at E3.5 diverged into two populations by the 64-cell stage, either as the GFP-high PrE or the GFP-low epiblasts (Epi). However in the GATA6-null blastocysts, the originally moderate GFP expression subsided in all ICM cells, indicating that the GATA6 protein is required to maintain its own promoter activity during PrE linage commitment. In embryonic stem cells, expressed GATA6 was shown to bind and activate the Gata6 promoter in PrE differentiation. Mutations of a conserved serine residue (S264) for Erk1/2 phosphorylation in GATA6 protein drastically impacted its ability to activate its own promoter. We conclude that phosphorylation of GATA6 by Erk1/2 compels exit from pluripotent state, and the phosphorylation propels a GATA6 positive feedback regulatory circuit to compel PrE differentiation. Our findings resolve the longstanding question on the dual requirements of GATA6 and Ras/MAPK pathway for PrE commitment of the pluripotent ICM.
Copyright © 2018 The Authors. Published by Elsevier Inc. All rights reserved.
-
WB
-
Mus musculus (House mouse)
-
Stem Cells and Developmental Biology
MicroRNA-145 regulates disabled-2 and Wnt3a expression in cardiomyocytes under hyperglycaemia.
In European Journal of Clinical Investigation on 1 January 2018 by Wang, B. W., Fang, W. J., et al.
MicroRNA-145 (miR-145) could protect cardiomyocyte apoptosis against oxidative stress and repair infarcted myocardium. Angiotensin II (Ang II), a pro-inflammatory cytokine could modulate myocardial remodelling. However, the role of hyperglycaemia on miR-145 expression in cardiomyocyte or diabetes is not known. The effect of Ang II on miR-145 expression under hyperglycaemia in cardiomyocytes remains unknown. We sought to investigate the effect of hyperglycaemia and Ang II on miR-145 expression in cardiomyocytes.
Rat cardiomyocytes were cultured under high glucose concentration (25 mmol/L), and streptozotocin-induced diabetic rats were established. TaqMan® MicroRNA real-time quantitative assay was used to quantitate miR-145.
Sustained high glucose concentration (hyperglycaemia) significantly decreased miR-145 expression in cardiomyocytes. Hyperglycaemia significantly increased Ang II mRNA expression and secretion from rat cardiomyocytes. Ang II suppressed miR-145 expression in cardiomyocytes. Hyperglycaemia increased Dab2 and decreased Wnt3a/ß-catenin expression in cardiomyocytes. Repression of miR-145 expression by Ang II resulted in increased Dab2 and decreased Wnt3a and ß-catenin expression under hyperglycaemia. In contrast, overexpression of miR-145 significantly decreased Dab2 mRNA and protein expression, whereas the mRNA and protein levels for Wnt3a and ß-catenin were significantly reduced in left ventricular myocardium from 5 days to 28 days in diabetic rats. The protein expression patterns of Dab2 and Wnt3a/ß-catenin in left ventricular myocardium of diabetic rats could be reversed upon treatment with valsartan.
Ang II downregulates miR-145 to regulate Dab2 and Wnt3a/ß-catenin expression in cardiomyocytes under high glucose concentration. Ang II plays a critical role in the regulation of miR-145 in cardiomyocytes under hyperglycaemic conditions.
© 2017 Stichting European Society for Clinical Investigation Journal Foundation.
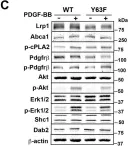
In eLife on 16 November 2017 by Xian, X., Ding, Y., et al.
Low-density lipoprotein receptor-related protein 1 (LRP1) is a multifunctional cell surface receptor with diverse physiological roles, ranging from cellular uptake of lipoproteins and other cargo by endocytosis to sensor of the extracellular environment and integrator of a wide range of signaling mechanisms. As a chylomicron remnant receptor, LRP1 controls systemic lipid metabolism in concert with the LDL receptor in the liver, whereas in smooth muscle cells (SMC) LRP1 functions as a co-receptor for TGFβ and PDGFRβ in reverse cholesterol transport and the maintenance of vascular wall integrity. Here we used a knockin mouse model to uncover a novel atheroprotective role for LRP1 in macrophages where tyrosine phosphorylation of an NPxY motif in its intracellular domain initiates a signaling cascade along an LRP1/SHC1/PI3K/AKT/PPARγ/LXR axis to regulate and integrate cellular cholesterol homeostasis through the expression of the major cholesterol exporter ABCA1 with apoptotic cell removal and inflammatory responses.
-
WB
-
Mus musculus (House mouse)
-
Immunology and Microbiology
In Developmental Dynamics : An Official Publication of the American Association of Anatomists on 1 July 2017 by Meng, Y., Cai, K. Q., et al.
Phosphatase and tensin homologue on chromosome 10 (Pten), a lipid phosphatase originally identified as a tumor-suppressor gene, regulates the phosphoinositol 3 kinase signaling pathway and impacts cell death and proliferation. Pten mutant embryos die at early stages of development, although the particular developmental deficiency and the mechanisms are not yet fully understood.
We analyzed Pten mutant embryos in detail and found that the formation of the proamniotic cavity is impaired. Embryoid bodies derived from Pten-null embryonic stem cells failed to undergo cavitation, reproducing the embryonic phenotype in vitro. Analysis of embryoid bodies and embryos revealed a role of Pten in the initiation of the focal point of the epithelial rosette that develops into the proamniotic lumen, and in establishment of epithelial polarity to transform the amorphous epiblast cells into a polarized epithelium.
We conclude that Pten is required for proamniotic cavity formation by establishing polarity for epiblast cells to form a rosette that expands into the proamniotic lumen, rather than facilitating apoptosis to create the cavity. Developmental Dynamics 246:517-530, 2017. © 2017 Wiley Periodicals, Inc.
© 2017 Wiley Periodicals, Inc.
-
IHC-IF
-
Mus musculus (House mouse)
-
Stem Cells and Developmental Biology
In Sci Rep on 17 May 2019 by Hocevar, B. A.
Fig.4.A

-
WB
-
Homo sapiens (Human)
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 6
In Elife on 16 November 2017 by Xian, X., Ding, Y., et al.
Fig.4.C

-
WB
-
Mus musculus (House mouse)
Collected and cropped from Elife by CiteAb, provided under a CC-BY license
Image 1 of 6
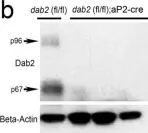
In Sci Rep on 25 October 2016 by Tao, W., Moore, R., et al.
Fig.3.B

-
WB
-
Mus musculus (House mouse)
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 6
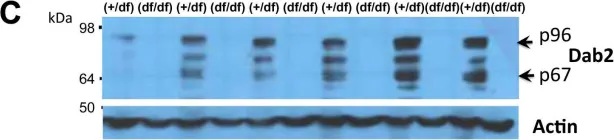
In PLoS One on 2 November 2014 by Tao, W., Moore, R., et al.
Fig.2.C

-
WB
-
Mus musculus (House mouse)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 6
In PLoS One on 2 November 2014 by Tao, W., Moore, R., et al.
Fig.2.A

-
WB
-
Mus musculus (House mouse)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 6
In PLoS One on 2 November 2014 by Tao, W., Moore, R., et al.
Fig.1.C

-
WB
-
Mus musculus (House mouse)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 6