Wnt signalling pathways play pivotal roles in development, homeostasis and human diseases, and are tightly regulated. We previously identified Tiki as a novel family of Wnt inhibitory proteases. Tiki proteins were predicted as type I transmembrane proteins and can act in both Wnt-producing and Wnt-responsive cells. Here, we characterize Tiki proteins as glycosylphosphatidylinositol (GPI)-anchored proteases. TIKI1/2 proteins are enriched on the detergent-resistant membrane microdomains and can be released from the plasma membrane by GPI-specific glycerophosphodiesterases GDE3 and GDE6, but not by GDE2. The GPI anchor determines the cellular localization of Tiki proteins and their regulation by GDEs, but not their inhibitory activity on Wnt signalling. Our study uncovered novel characteristics and potential regulations of the Tiki family proteases.
© 2022 Federation of European Biochemical Societies.
Product Citations: 21
Tiki proteins are glycosylphosphatidylinositol-anchored proteases.
In FEBS Letters on 1 April 2022 by Li, M., Zheng, J., et al.
In Hypertension on 1 June 2021 by Liao, J., Lu, W., et al.
In Oncology Letters on 1 February 2020 by Sun, J., Lu, Y., et al.
Head and neck squamous cell carcinoma (HNSCC) is the sixth most frequent malignancy with a 5-year survival rate of 54%. Therefore, disease management improvement is required. The present study aimed to assess the role of caveolin-1 (Cav-1) in the metastasis of head and neck tumor cells. Short hairpin RNA was used to silence Cav-1 expression in Tu686 cells. Proliferation, migration, invasion, morphology and the levels of effector proteins were assessed in cells. Upon Cav-1 silencing, E-cadherin levels were decreased, while vimentin levels were significantly increased. Cell migration, quantified by wound healing and Transwell assays, was significantly increased. Meanwhile, Cav-1 and transforming growth factor β1 (TGF-β1) receptor were identified to be co-localized. In addition, Cav-1-knockdown resulted in increased phosphorylation of SMAD family member 2 (P<0.05), a downstream effector of TGF-β signaling. In addition, there was a mutual regulation, with increasing TGF-β1 levels leading to a dose-dependent decrease of Cav-1 expression levels (P<0.05). These findings indicate that Cav-1 inhibits cell metastasis in HNSCC, suggesting the involvement of the TGF-β signaling pathway.
Copyright: © Sun et al.
-
IF
-
ICC-IF
-
Homo sapiens (Human)
-
Cancer Research
Clostridium difficile Toxin A Undergoes Clathrin-Independent, PACSIN2-Dependent Endocytosis.
In PLoS Pathogens on 1 December 2016 by Chandrasekaran, R., Kenworthy, A. K., et al.
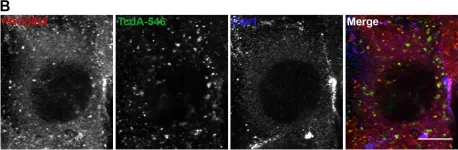
Clostridium difficile infection affects a significant number of hospitalized patients in the United States. Two homologous exotoxins, TcdA and TcdB, are the major virulence factors in C. difficile pathogenesis. The toxins are glucosyltransferases that inactivate Rho family-GTPases to disrupt host cellular function and cause fluid secretion, inflammation, and cell death. Toxicity depends on receptor binding and subsequent endocytosis. TcdB has been shown to enter cells by clathrin-dependent endocytosis, but the mechanism of TcdA uptake is still unclear. Here, we utilize a combination of RNAi-based knockdown, pharmacological inhibition, and cell imaging approaches to investigate the endocytic mechanism(s) that contribute to TcdA uptake and subsequent cytopathic and cytotoxic effects. We show that TcdA uptake and cellular intoxication is dynamin-dependent but does not involve clathrin- or caveolae-mediated endocytosis. Confocal microscopy using fluorescently labeled TcdA shows significant colocalization of the toxin with PACSIN2-positive structures in cells during entry. Disruption of PACSIN2 function by RNAi-based knockdown approaches inhibits TcdA uptake and toxin-induced downstream effects in cells indicating that TcdA entry is PACSIN2-dependent. We conclude that TcdA and TcdB utilize distinct endocytic mechanisms to intoxicate host cells.
-
IF
-
ICC-IF
-
Homo sapiens (Human)
-
Mus musculus (House mouse)
-
Immunology and Microbiology
A2A adenosine receptor modulates drug efflux transporter P-glycoprotein at the blood-brain barrier.
In The Journal of Clinical Investigation on 2 May 2016 by Kim, D. G. & Bynoe, M. S.
The blood-brain barrier (BBB) protects the brain from toxic substances within the peripheral circulation. It maintains brain homeostasis and is a hurdle for drug delivery to the CNS to treat neurodegenerative diseases, including Alzheimer's disease and brain tumors. The drug efflux transporter P-glycoprotein (P-gp) is highly expressed on brain endothelial cells and blocks the entry of most drugs delivered to the brain. Here, we show that activation of the A2A adenosine receptor (AR) with an FDA-approved A2A AR agonist (Lexiscan) rapidly and potently decreased P-gp expression and function in a time-dependent and reversible manner. We demonstrate that downmodulation of P-gp expression and function coincided with chemotherapeutic drug accumulation in brains of WT mice and in primary mouse and human brain endothelial cells, which serve as in vitro BBB models. Lexiscan also potently downregulated the expression of BCRP1, an efflux transporter that is highly expressed in the CNS vasculature and other tissues. Finally, we determined that multiple pathways, including MMP9 cleavage and ubiquitinylation, mediated P-gp downmodulation. Based on these data, we propose that A2A AR activation on BBB endothelial cells offers a therapeutic window that can be fine-tuned for drug delivery to the brain and has potential as a CNS drug-delivery technology.
-
Cardiovascular biology
-
Genetics
In PLoS Pathog on 1 December 2016 by Chandrasekaran, R., Kenworthy, A. K., et al.
Fig.4.B

-
ICC-IF
-
Mus musculus (House mouse)
Collected and cropped from PLoS Pathog by CiteAb, provided under a CC-BY license
Image 1 of 1