Advanced pathological and genetic approaches have revealed that mutations in fused in sarcoma/translated in liposarcoma (FUS/TLS), which is pivotal for DNA repair, alternative splicing, translation and RNA transport, cause familial amyotrophic lateral sclerosis (ALS). The generation of suitable animal models for ALS is essential for understanding its pathogenesis and developing therapies. Therefore, we used CRISPR-Cas9 to generate FUS-ALS mutation in the non-classical nuclear localization signal (NLS), H517D (mouse position: H509D) and genome-edited mice. Fus WT/H509D mice showed progressive motor impairment (accelerating rotarod and DigiGait system) with age, which was associated with the loss of motor neurons and disruption of the nuclear lamina and nucleoporins and DNA damage in spinal cord motor neurons. We confirmed the validity of our model by showing that nuclear lamina and nucleoporin disruption were observed in lower motor neurons differentiated from patient-derived human induced pluripotent stem cells (hiPSC-LMNs) with FUS-H517D and in the post-mortem spinal cord of patients with ALS. RNA sequence analysis revealed that most nuclear lamina and nucleoporin-linking genes were significantly decreased in FUS-H517D hiPSC-LMNs. This evidence suggests that disruption of the nuclear lamina and nucleoporins is crucial for ALS pathomechanisms. Combined with patient-derived hiPSC-LMNs and autopsy samples, this mouse model might provide a more reliable understanding of ALS pathogenesis and might aid in the development of therapeutic strategies.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For commercial re-use, please contact reprints@oup.com for reprints and translation rights for reprints. All other permissions can be obtained through our RightsLink service via the Permissions link on the article page on our site—for further information please contact journals.permissions@oup.com.
Product Citations: 81
In Brain on 4 November 2024 by Okada, K., Ito, D., et al.
-
Neuroscience
Annulate Lamellae biogenesis is essential for nuclear pore function
Preprint on BioRxiv : the Preprint Server for Biology on 9 October 2024 by Lin, J., Agote-Arán, A., et al.
Nuclear pore complexes (NPCs), large protein assemblies embedded into the nuclear envelope (NE), are crucial for bidirectional transport between the nucleus and cytoplasm, a process often disrupted in human diseases. Besides their presence within the NE, NPCs are also found in stacked cytoplasmic endoplasmic reticulum (ER) membranes called annulate lamellae (AL) 1,2 . Despite being discovered in the mid-20th century 3 , the function and biogenesis mechanisms of AL have remained largely mysterious. While AL were thought to be restricted to germ, embryonic and malignant cells 4–12 , we find that AL also exist in the cytoplasm of somatic cells under normal physiological conditions and that they can expand upon specific stimuli. We show that AL merge with the NE, supplying the nucleus with new pores which maintains nuclear pore function and nuclear growth during early interphase. NPC protein RanBP2 (Nup358) and ER-associated Climp63 (CKAP4) trigger AL assembly and their NE-integration. The N-terminal phenylalanine-glycine (FG) repeats of RanBP2 drive the oligomerization of Y-complexes (the NPC outer ring units), and AL-NPCs formation and Climp63 ensures the localization of AL-NPCs to ER sheets and their fusion with the nucleus. These findings uncover a fundamental mechanism of AL biogenesis and highlight the critical role of cytosolic NPCs in the nuclear function and mammalian cellular homeostasis.
-
Homo sapiens (Human)
Nup358 restricts ER-mitochondria connectivity by modulating mTORC2/Akt/GSK3β signalling.
In EMBO Reports on 1 October 2024 by Kalarikkal, M., Saikia, R., et al.
ER-mitochondria contact sites (ERMCSs) regulate processes, including calcium homoeostasis, energy metabolism and autophagy. Previously, it was shown that during growth factor signalling, mTORC2/Akt gets recruited to and stabilizes ERMCSs. Independent studies showed that GSK3β, a well-known Akt substrate, reduces ER-mitochondria connectivity by disrupting the VAPB-PTPIP51 tethering complex. However, the mechanisms that regulate ERMCSs are incompletely understood. Here we find that annulate lamellae (AL), relatively unexplored subdomains of ER enriched with a subset of nucleoporins, are present at ERMCSs. Depletion of Nup358, an AL-resident nucleoporin, results in enhanced mTORC2/Akt activation, GSK3β inhibition and increased ERMCSs. Depletion of Rictor, a mTORC2-specific subunit, or exogenous expression of GSK3β, was sufficient to reverse the ERMCS-phenotype in Nup358-deficient cells. We show that growth factor-mediated activation of mTORC2 requires the VAPB-PTPIP51 complex, whereas, Nup358's association with this tether restricts mTORC2/Akt signalling and ER-mitochondria connectivity. Expression of a Nup358 fragment that is sufficient for interaction with the VAPB-PTPIP51 complex suppresses mTORC2/Akt activation and disrupts ERMCSs. Collectively, our study uncovers a novel role for Nup358 in controlling ERMCSs by modulating the mTORC2/Akt/GSK3β axis.
© 2024. The Author(s).
-
Cell Biology
UBAP2L ensures homeostasis of nuclear pore complexes at the intact nuclear envelope.
In The Journal of Cell Biology on 1 July 2024 by Liao, Y., Andronov, L., et al.
Assembly of macromolecular complexes at correct cellular sites is crucial for cell function. Nuclear pore complexes (NPCs) are large cylindrical assemblies with eightfold rotational symmetry, built through hierarchical binding of nucleoporins (Nups) forming distinct subcomplexes. Here, we uncover a role of ubiquitin-associated protein 2-like (UBAP2L) in the assembly and stability of properly organized and functional NPCs at the intact nuclear envelope (NE) in human cells. UBAP2L localizes to the nuclear pores and facilitates the formation of the Y-complex, an essential scaffold component of the NPC, and its localization to the NE. UBAP2L promotes the interaction of the Y-complex with POM121 and Nup153, the critical upstream factors in a well-defined sequential order of Nups assembly onto NE during interphase. Timely localization of the cytoplasmic Nup transport factor fragile X-related protein 1 (FXR1) to the NE and its interaction with the Y-complex are likewise dependent on UBAP2L. Thus, this NPC biogenesis mechanism integrates the cytoplasmic and the nuclear NPC assembly signals and ensures efficient nuclear transport, adaptation to nutrient stress, and cellular proliferative capacity, highlighting the importance of NPC homeostasis at the intact NE.
© 2024 Liao et al.
-
Homo sapiens (Human)
-
Cell Biology
In Journal of Extracellular Biology on 1 July 2024 by Vassileff, N., Spiers, J. G., et al.
Neuroinflammation is initiated through microglial activation and cytokine release which can be induced through lipopolysaccharide treatment (LPS) leading to a transcriptional cascade culminating in the differential expression of target proteins. These differentially expressed proteins can then be packaged into extracellular vesicles (EVs), a form of cellular communication, further propagating the neuroinflammatory response over long distances. Despite this, the EV proteome in the brain, following LPS treatment, has not been investigated. Brain tissue and brain derived EVs (BDEVs) isolated from the cortex of LPS-treated mice underwent thorough characterisation to meet the minimal information for studies of extracellular vesicles guidelines before undergoing mass spectrometry analysis to identify the differentially expressed proteins. Fourteen differentially expressed proteins were identified in the LPS brain tissue samples compared to the controls and 57 were identified in the BDEVs isolated from the LPS treated mice compared to the controls. This included proteins associated with the initiation of the inflammatory response, epigenetic regulation, and metabolism. These results allude to a potential link between small EV cargo and early inflammatory signalling.
© 2024 The Author(s). Journal of Extracellular Biology published by Wiley Periodicals LLC on behalf of International Society for Extracellular Vesicles.
-
Biochemistry and Molecular biology
-
Cell Biology
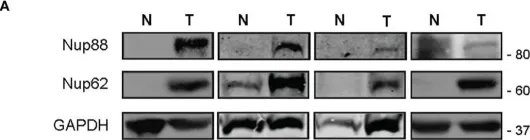
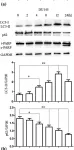
In Front Oncol on 28 February 2023 by Singh, U., Bindra, D., et al.
Fig.1.A

-
WB
-
Collected and cropped from Front Oncol by CiteAb, provided under a CC-BY license
Image 1 of 12
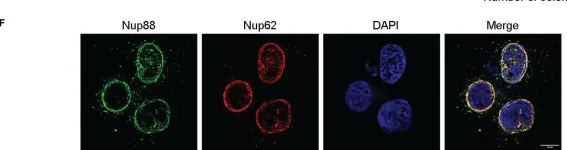
In Front Oncol on 28 February 2023 by Singh, U., Bindra, D., et al.
Fig.2.F

-
ICC-IF
-
Collected and cropped from Front Oncol by CiteAb, provided under a CC-BY license
Image 1 of 12
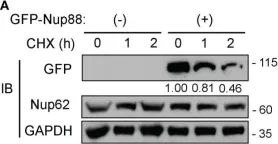
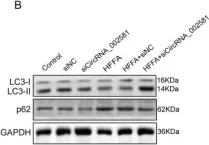
In Front Oncol on 28 February 2023 by Singh, U., Bindra, D., et al.
Fig.5.A

-
WB
-
Collected and cropped from Front Oncol by CiteAb, provided under a CC-BY license
Image 1 of 12
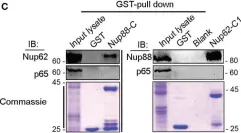
In Front Oncol on 28 February 2023 by Singh, U., Bindra, D., et al.
Fig.6.C

-
WB
-
Collected and cropped from Front Oncol by CiteAb, provided under a CC-BY license
Image 1 of 12
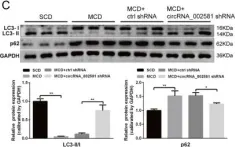
In Cell Death Dis on 13 February 2020 by Jin, X., Gao, J., et al.
Fig.5.C

-
WB
-
Collected and cropped from Cell Death Dis by CiteAb, provided under a CC-BY license
Image 1 of 12
In Cell Death Dis on 13 February 2020 by Jin, X., Gao, J., et al.
Fig.6.B

-
WB
-
Collected and cropped from Cell Death Dis by CiteAb, provided under a CC-BY license
Image 1 of 12
In Int Urol Nephrol on 1 April 2018 by Wang, Q., He, W. Y., et al.
Fig.1.A

-
WB
-
Homo sapiens (Human)
Collected and cropped from Int Urol Nephrol by CiteAb, provided under a CC-BY license
Image 1 of 12
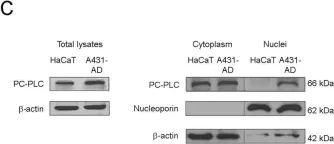
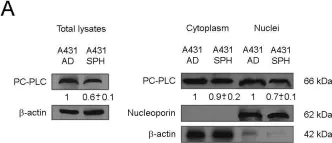
In PLoS One on 25 September 2015 by Cecchetti, S., Bortolomai, I., et al.
Fig.1.C

-
WB
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 12
In PLoS One on 25 September 2015 by Cecchetti, S., Bortolomai, I., et al.
Fig.4.A

-
WB
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 12
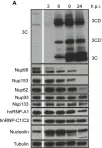
In PLoS One on 21 August 2013 by Walker, E. J., Younessi, P., et al.
Fig.1.A

-
WB
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 12
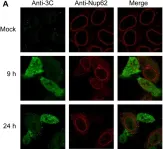
In PLoS One on 21 August 2013 by Walker, E. J., Younessi, P., et al.
Fig.2.A

-
ICC-IF
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 12
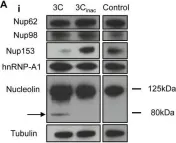
In PLoS One on 21 August 2013 by Walker, E. J., Younessi, P., et al.
Fig.3.A

-
WB
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 12