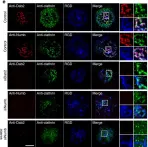
Ca2+ release-activated Ca2+ (CRAC) channels are highly Ca2+ selective plasma membrane channels formed by the hexameric assembly of Orai subunits, with a predominant role for Orai1. Two Orai1 variants have been identified, Orai1α, which comprises 301 amino acids, and a short variant, Orai1β, lacking the first N-terminal 63 or 71 amino acids; however, little is known about their possible heteromerization to form CRAC channels. Here we show that Orai1α and Orai1β exhibit different lipid raft distributions in resting cells when expressed individually, likely due to the presence of a caveolin-binding domain exclusively in Orai1α. However, when both variants are co-expressed, they show a similar distribution predominantly in the lipid raft domains, indicating potential interaction between the two Orai1 forms.
A lipid raft isolation protocol in combination with Western blotting assay was conducted to detect the expression of each Orai1 variants in the isolated membrane fractions. Ca2+ mobilization was determined using fura-2 and G-GECO1.2 fused to Orai1α fluorescence. Evidence of physical interaction between both Orai1 variants was provided using co-immunoprecipitation, APEX2 peroxidase-catalyzed proximity labeling, Förster resonance energy transfer (FRET) and super-resolution microscopy.
Our results indicate that Orai1α and Orai1β exhibit different lipid raft partitioning in resting cells when expressed individually, likely attributed to the presence of a caveolin-binding domain in Orai1α. However, when both variants are co-expressed, they show a similar distribution predominantly in the lipid raft domains, indicating potential interaction between the two Orai1 forms. Expression of a dominant-negative Orai1β mutant has been found to interfere with Orai1α-mediated Ca2+ entry. Using co-immunoprecipitation, APEX2 peroxidase-catalyzed proximity labeling, Förster resonance energy transfer (FRET) and super-resolution microscopy our results indicate that there is certain interaction between Orai1α and Orai1β although both variants form mostly independent channels.
Our results indicate that while Orai1α and Orai1β mostly form separate CRAC channels, a small subset of both Orai1 variants combine to form heteromeric channels. These findings provide new insights on the nature of CRAC channels.
© 2025. The Author(s).
Product Citations: 130
A subset of Orai1α and Orai1β subunits heteromerizes to form CRAC channels.
In Cell Communication and Signaling : CCS on 2 June 2025 by López, J. J., Jardín, I., et al.
-
WB
-
Endocrinology and Physiology
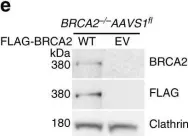
In JCO Precision Oncology on 1 June 2024 by Vanoli, F., Song, E., et al.
Targeted therapy in translocation-associated sarcomas has been limited to oncogenic activation of tyrosine kinases or ligands while gene fusions resulting in aberrant expression of transcription factors have been notoriously difficult to target. Moreover, secondary genetic alterations in sarcomas driven by translocations are uncommon, comprising mostly alterations in tumor suppressor genes (TP53, CDKN2A/B). Our study was triggered by an index patient showing a dramatic clinical response by targeting the secondary BRAF V600E mutation in a metastatic angiomatoid fibrous histiocytoma (AFH) harboring the typical EWSR1::CREB1 fusion.
The patient, a 28-year-old female, was diagnosed with an AFH of the thigh and followed a highly aggressive clinical course, with rapid multifocal local recurrence within a year and widespread distant metastases (adrenal, bone, liver, lung). The tumor showed characteristic morphologic features, with histiocytoid cells intermixed with hemorrhagic cystic spaces and lymphoid aggregates. In addition to the pathognomonic EWSR1::CREB1 fusion, targeted DNA sequencing revealed in both primary and adrenal metastatic sites a hot spot BRAF V600E mutation and a CDKN2A/B deletion. Accordingly, the patient was treated with a BRAF-MEK inhibitor combination (encorafenib/binimetinib) showing an excellent but short-lived response.
Using a CRISPR-Cas9 approach, we introduced the BRAF c.1799 T>A point mutation in human embryonic stem (hES) cells harboring a conditional EWSR1 (exon7)::CREB1 (exon7) translocation and further differentiated to mesenchymal progenitors (hES-MP) before fusion expression. The cells maintained the fusion transcript expression and the AFH core gene signature while responding to treatment with encorafenib and binimetinib.
These results highlight that additional targeted DNA NGS in chemotherapy-resistant translocation-associated sarcomas may reveal actionable oncogenic drivers occurring as secondary genetic events during disease progression.
New Generation Self-Replicating RNA Vaccines Derived from Pestivirus Genome.
In Methods in Molecular Biology (Clifton, N.J.) on 30 May 2024 by Démoulins, T., Techakriengkrai, N., et al.
While mRNA vaccines have shown their worth, they have the same failing as inactivated vaccines, namely they have limited half-life, are non-replicating, and therefore limited to the size of the vaccine payload for the amount of material translated. New advances averting these problems are combining replicon RNA (RepRNA) technology with nanotechnology. RepRNA are large self-replicating RNA molecules (typically 12-15 kb) derived from viral genomes defective in at least one essential structural protein gene. They provide sustained antigen production, effectively increasing vaccine antigen payloads over time, without the risk of producing infectious progeny. The major limitations with RepRNA are RNase-sensitivity and inefficient uptake by dendritic cells (DCs), which need to be overcome for efficacious RNA-based vaccine design. We employed biodegradable delivery vehicles to protect the RepRNA and promote DC delivery. Condensing RepRNA with polyethylenimine (PEI) and encapsulating RepRNA into novel Coatsome-replicon vehicles are two approaches that have proven effective for delivery to DCs and induction of immune responses in vivo.
© 2024. The Author(s), under exclusive license to Springer Science+Business Media, LLC, part of Springer Nature.
-
Biochemistry and Molecular biology
-
Genetics
-
Immunology and Microbiology
Glutathione-dependent depalmitoylation of phospholemman by peroxiredoxin 6.
In Cell Reports on 27 February 2024 by Howie, J., Tulloch, L. B., et al.
Phospholemman (PLM) regulates the cardiac sodium pump: PLM phosphorylation activates the pump whereas PLM palmitoylation inhibits its activity. Here, we show that the anti-oxidant protein peroxiredoxin 6 (Prdx6) interacts with and depalmitoylates PLM in a glutathione-dependent manner. Glutathione loading cells acutely reduce PLM palmitoylation; glutathione depletion significantly increases PLM palmitoylation. Prdx6 silencing abolishes these effects, suggesting that PLM can be depalmitoylated by reduced Prdx6. In vitro, only recombinant Prdx6, among several peroxiredoxin isoforms tested, removes palmitic acid from recombinant palmitoylated PLM. The broad-spectrum depalmitoylase inhibitor palmostatin B prevents Prdx6-dependent PLM depalmitoylation in cells and in vitro. Our data suggest that Prdx6 is a thioesterase that can depalmitoylate proteins by nucleophilic attack via its reactive thiol, linking PLM palmitoylation and hence sodium pump activity to cellular glutathione status. We show that protein depalmitoylation can occur via a catalytic cysteine in which substrate specificity is determined by a protein-protein interaction.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
In ENeuro on 22 February 2024 by Pelz, L., Dossou, L., et al.
The type I transmembrane protein BT-IgSF is predominantly localized in the brain and testes. It belongs to the CAR subgroup of Ig cell adhesion proteins, that are hypothesized to regulate connexin expression or localization. Here, we studied the putative link between BT-IgSF and connexins in astrocytes, ependymal cells and neurons of the mouse. Global knockout of BT-IgSF caused an increase in the clustering of connexin43 (Gja1), but not of connexin30 (Gjb6), on astrocytes and ependymal cells. Additionally, knockout animals displayed reduced expression levels of connexin43 protein in the cortex and hippocampus. Importantly, analysis of biocytin spread in hippocampal or cortical slices from mature mice of either sex revealed a decrease in astrocytic cell-cell coupling in the absence of BT-IgSF. Blocking either protein biosynthesis or proteolysis showed that the lysosomal pathway increased connexin43 degradation in astrocytes. Localization of connexin43 in subcellular compartments was not impaired in astrocytes of BT-IgSF mutants. In contrast to connexin43 the localization and expression of connexin36 (Gjd2) on neurons was not affected by the absence of BT-IgSF. Overall, our data indicate that the IgCAM BT-IgSF is essential for correct gap junction-mediated astrocyte-to-astrocyte cell communication.Significance Statement Astrocytes regulate a variety of physiological processes in the developing and adult brain that are essential for proper brain function. Astrocytes form extensive networks in the brain and communicate via gap junctions. Disruptions of gap junction coupling are found in several diseases such as neurodegeneration or epilepsy. Here, we demonstrate that the cell adhesion protein BT-IgSF is essential for gap junction mediated coupling between astrocytes in the cortex and hippocampus.
Copyright © 2024 Pelz et al.
-
Neuroscience
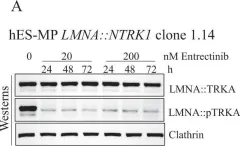
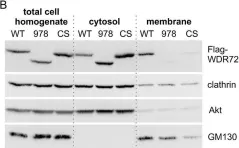
In Oncogenesis on 17 February 2023 by Vanoli, F., Herviou, L., et al.
Fig.4.A

-
WB
-
Collected and cropped from Oncogenesis by CiteAb, provided under a CC-BY license
Image 1 of 20
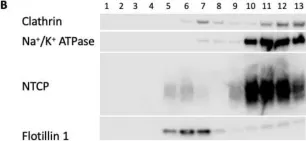
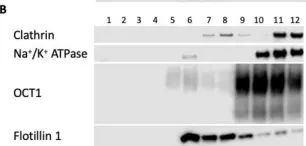
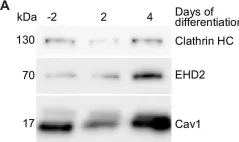
In Int J Mol Sci on 30 July 2022 by Idowu, J. Y. & Hagenbuch, B.
Fig.9.B

-
WB
-
Homo sapiens (Human)
Collected and cropped from Int J Mol Sci by CiteAb, provided under a CC-BY license
Image 1 of 20
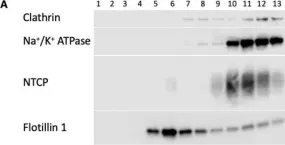
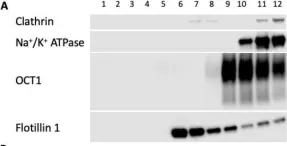
In Int J Mol Sci on 30 July 2022 by Idowu, J. Y. & Hagenbuch, B.
Fig.9.A

-
WB
-
Homo sapiens (Human)
Collected and cropped from Int J Mol Sci by CiteAb, provided under a CC-BY license
Image 1 of 20
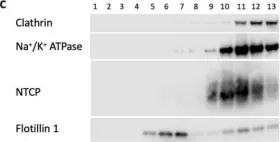
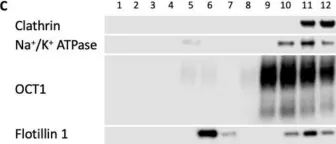
In Int J Mol Sci on 30 July 2022 by Idowu, J. Y. & Hagenbuch, B.
Fig.9.C

-
WB
-
Homo sapiens (Human)
Collected and cropped from Int J Mol Sci by CiteAb, provided under a CC-BY license
Image 1 of 20
In Int J Mol Sci on 30 July 2022 by Idowu, J. Y. & Hagenbuch, B.
Fig.10.B

-
WB
-
Homo sapiens (Human)
Collected and cropped from Int J Mol Sci by CiteAb, provided under a CC-BY license
Image 1 of 20
In Int J Mol Sci on 30 July 2022 by Idowu, J. Y. & Hagenbuch, B.
Fig.10.A

-
WB
-
Homo sapiens (Human)
Collected and cropped from Int J Mol Sci by CiteAb, provided under a CC-BY license
Image 1 of 20
In Int J Mol Sci on 30 July 2022 by Idowu, J. Y. & Hagenbuch, B.
Fig.10.C

-
WB
-
Homo sapiens (Human)
Collected and cropped from Int J Mol Sci by CiteAb, provided under a CC-BY license
Image 1 of 20
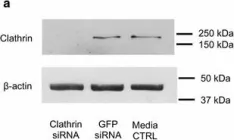
In Sci Rep on 17 March 2022 by Husein, D., Alamoudi, A., et al.
Fig.6.B

-
WB
-
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 20
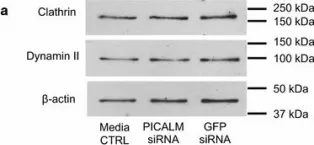
In Elife on 4 May 2020 by Hubert, M., Larsson, E., et al.
Fig.5.A

-
WB
-
Collected and cropped from Elife by CiteAb, provided under a CC-BY license
Image 1 of 20
In Nat Commun on 13 September 2017 by Feng, W. & Jasin, M.
Fig.1.E

-
WB
-
Homo sapiens (Human)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 20
In BMC Neurosci on 18 July 2016 by Thomas, R. S., Henson, A., et al.
Fig.8.A

-
WB
-
Collected and cropped from BMC Neurosci by CiteAb, provided under a CC-BY license
Image 1 of 20
In BMC Neurosci on 18 July 2016 by Thomas, R. S., Henson, A., et al.
Fig.3.A

-
WB
-
Collected and cropped from BMC Neurosci by CiteAb, provided under a CC-BY license
Image 1 of 20
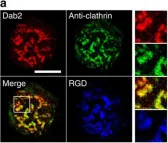
In Nat Commun on 28 October 2015 by Yu, C. H., Rafiq, N. B., et al.
Fig.5.E

-
ICC-IF
-
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 20
In Nat Commun on 28 October 2015 by Yu, C. H., Rafiq, N. B., et al.
Fig.5.A

-
IHC-IF
-
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 20
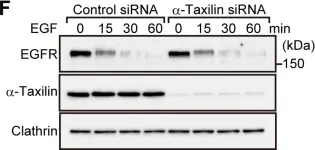
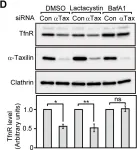
In PLoS One on 3 April 2014 by Sakane, H., Horii, Y., et al.
Fig.1.F

-
WB
-
Homo sapiens (Human)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 20
In PLoS One on 3 April 2014 by Sakane, H., Horii, Y., et al.
Fig.1.D

-
WB
-
Homo sapiens (Human)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 20
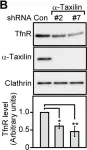
In PLoS One on 3 April 2014 by Sakane, H., Horii, Y., et al.
Fig.1.B

-
WB
-
Homo sapiens (Human)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 20
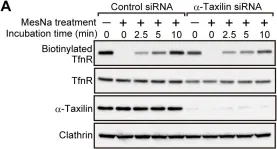
In PLoS One on 3 April 2014 by Sakane, H., Horii, Y., et al.
Fig.2.A

-
WB
-
Homo sapiens (Human)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 20
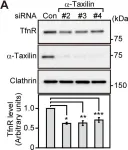
In PLoS One on 3 April 2014 by Sakane, H., Horii, Y., et al.
Fig.1.A

-
WB
-
Homo sapiens (Human)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 20
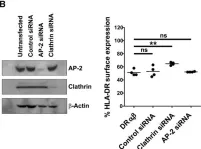
In Eur J Immunol on 1 April 2013 by Jackson, N. P., Kang, Y. H., et al.
Fig.2.B

-
WB
-
Homo sapiens (Human)
Collected and cropped from Eur J Immunol by CiteAb, provided under a CC-BY license
Image 1 of 20