Brain activity relies on a steady supply of blood glucose. Astrocytes express glucose transporter 1 (GLUT1), considered their primary route for glucose uptake to sustain metabolic and antioxidant support for neurons. While GLUT1 deficiency causes severe developmental impairments, its role in adult astrocytes remains unclear. Here, we show that astrocytes and neurons tolerate the inducible, astrocyte-specific deletion of GLUT1 in adulthood. Sensorimotor and memory functions remain intact in male GLUT1 cKO mice, indicating that GLUT1 loss does not impair behavior. Despite GLUT1 loss, two-photon glucose sensor imaging reveals that astrocytes maintain normal resting glucose levels but exhibit a more than two-fold increase in glucose consumption, indicating enhanced metabolic activity. Notably, male GLUT1 cKO mice display reduced infarct volumes following stroke, suggesting a neuroprotective effect of increased astrocytic glucose metabolism. Our findings reveal metabolic adaptability in astrocytes, ensuring glucose uptake and neuronal support despite the absence of their primary transporter.
© 2025. The Author(s).
Product Citations: 73
Astrocytic GLUT1 deletion in adult mice enhances glucose metabolism and resilience to stroke.
In Nature Communications on 6 May 2025 by Thieren, L., Zanker, H. S., et al.
-
Biochemistry and Molecular biology
-
Cardiovascular biology
-
Cell Biology
Immunohistochemistry and Spatial Density of Müller Cells in the Human Fovea.
In Investigative Ophthalmology & Visual Science on 3 February 2025 by Masri, R. A., Greferath, U., et al.
Previous evidence indicates that molecular properties of foveal Müller cells are different from those in the peripheral retina. Here we aimed to characterize Müller cells in the human fovea (including the foveal floor) with specific focus on their spatial density and immunohistochemistry.
Human retinas were obtained postmortem from male and female donors with no known eye disease (aged 31-56 years) or after exenteration (one 75-year-old patient with no retinal disease and one 86-year-old patient with reticular pseudodrusen). Vertical sections through the macula were processed for immunofluorescence using antibodies against cellular retinaldehyde binding protein (CRALBP), glutamine synthetase (GS), glial fibrillary acidic protein (GFAP), transient receptor potential vanilloid 4 (TRPV4), excitatory amino acid transporter 4 (EAAT4), calbindin, and RNA-binding protein with multiple splicing. Sections were imaged using high-resolution, multichannel confocal microscopy.
Immunofluorescence for CRALBP and GS was found in Müller cells, including their processes throughout the retina. GFAP expression was found in astrocytes outside the fovea and in some foveal somas. Müller cell nuclei had a peak density of about 35,000 cells/mm2 at 500 µm eccentricity. Calbindin was coexpressed with CRALBP in up to 96% of Müller cells in the fovea, but at eccentricities beyond about 1.5 mm calbindin was not expressed by Müller cells. Conversely, calbindin expression in cone photoreceptors was absent in foveal but present in peripheral retina.
This study supports the hypothesis that Müller cells in the macula have distinct structural, functional, and immunohistochemical properties compared to their peripheral counterparts.
-
Neuroscience
Evolutionary conservation of VSX2 super-enhancer modules in retinal development.
In Development (Cambridge, England) on 1 July 2024 by Honnell, V., Sweeney, S., et al.
Super-enhancers (SEs) are expansive regions of genomic DNA that regulate the expression of genes involved in cell identity and cell fate. We recently identified developmental stage- and cell type-specific modules within the murine Vsx2 SE. Here, we show that the human VSX2 SE modules have similar developmental stage- and cell type-specific activity in reporter gene assays. By inserting the human sequence of one VSX2 SE module into a mouse with microphthalmia, eye size was rescued. To understand the function of these SE modules during human retinal development, we deleted individual modules in human embryonic stem cells and generated retinal organoids. Deleting one module results in small organoids, recapitulating the small-eyed phenotype of mice with microphthalmia, while deletion of the other module led to disruptions in bipolar neuron development. This prototypical SE serves as a model for understanding developmental stage- and cell type-specific effects of neurogenic transcription factors with complex expression patterns. Moreover, by elucidating the gene regulatory mechanisms, we can begin to examine how dysregulation of these mechanisms contributes to phenotypic diversity and disease.
© 2024. Published by The Company of Biologists Ltd.
-
Mus musculus (House mouse)
-
Stem Cells and Developmental Biology
Sbp2l contributes to oligodendrocyte maturation through translational control in Tcf7l2 signaling.
In IScience on 15 December 2023 by Yugami, M., Hayakawa-Yano, Y., et al.
Oligodendrocytes (OLs) are the myelin-forming cells in the CNS that support neurons through the insulating sheath of axons. This unique feature and developmental processes are achieved by extrinsic and intrinsic gene expression programs, where RNA-binding proteins can contribute to dynamic and fine-tuned post-transcriptional regulation. Here, we identified SECIS-binding protein 2-like (Sbp2l), which is specifically expressed in OLs by integrated transcriptomics. Histological analysis revealed that Sbp2l is a molecular marker of OL maturation. Sbp2l knockdown (KD) led to suppression of matured OL markers, but not a typical selenoprotein, Gpx4. Transcriptome analysis demonstrated that Sbp2l KD decreased cholesterol-biosynthesis-related genes regulated by Tcf7l2 transcription factor. Indeed, we confirmed the downregulation of Tcf7l2 protein without changing its mRNA in Sbp2l KD OPCs. Furthermore, Sbp2l KO mice showed the decrease of Tcf7l2 protein and deficiency of OL maturation. These results suggest that Sbp2l contributes to OL maturation by translational control of Tcf7l2.
© 2023 The Author(s).
-
Biochemistry and Molecular biology
-
Neuroscience
Osteopontin drives retinal ganglion cell resiliency in glaucomatous optic neuropathy.
In Cell Reports on 26 September 2023 by Zhao, M., Toma, K., et al.
Chronic neurodegeneration and acute injuries lead to neuron losses via diverse processes. We compared retinal ganglion cell (RGC) responses between chronic glaucomatous conditions and the acute injury model. Among major RGC subclasses, αRGCs and intrinsically photosensitive RGCs (ipRGCs) preferentially survive glaucomatous conditions, similar to findings in the retina subject to axotomy. Focusing on an αRGC intrinsic factor, Osteopontin (secreted phosphoprotein 1 [Spp1]), we found an ectopic neuronal expression of Osteopontin (Spp1) in other RGCs subject to glaucomatous conditions. This contrasted with the Spp1 downregulation subject to axotomy. αRGC-specific Spp1 elimination led to significant αRGC loss, diminishing their resiliency. Spp1 overexpression led to robust neuroprotection of susceptible RGC subclasses under glaucomatous conditions. In contrast, Spp1 overexpression did not significantly protect RGCs subject to axotomy. Additionally, SPP1 marked adult human RGC subsets with large somata and SPP1 expression in the aqueous humor correlated with glaucoma severity. Our study reveals Spp1's role in mediating neuronal resiliency in glaucoma.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
-
Neuroscience
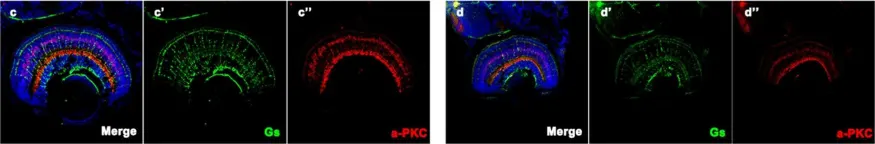
In Hum Genet on 1 October 2019 by Xiao, X., Sun, W., et al.
Fig.5.C

-
IHC-IF
-
Danio rerio (Zebrafish)
Collected and cropped from Hum Genet by CiteAb, provided under a CC-BY license
Image 1 of 3
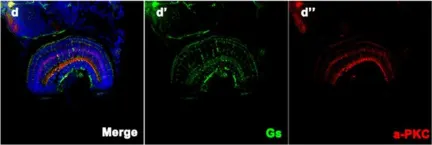
In Hum Genet on 1 October 2019 by Xiao, X., Sun, W., et al.
Fig.5.D

-
IHC-IF
-
Danio rerio (Zebrafish)
Collected and cropped from Hum Genet by CiteAb, provided under a CC-BY license
Image 1 of 3
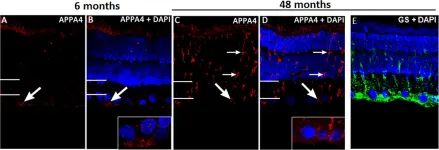
In PLoS One on 13 August 2015 by Du, L. Y., Chang, L. Y., et al.
Fig.2.A

-
IHC
-
Rattus norvegicus (Rat)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 3