Extracellular vesicles (EVs) hold great promise as circulating biomarkers for diagnostic and therapeutic approaches. Thus, many research groups world-wide investigate important aspects of EVs including their biology and medical significance. For this, a large number of procedures and protocols has been established making it difficult to almost impossible to compare and replicate results. Consequently, diagnostic tests remain problematic to interpret, mainly because the use of reliable reference EVs as a qualified standard has not yet gained widespread acceptance. Beyond doubt, such reference EVs are key to assess EV preparations quantitatively and to establish robust quality control processes to ensure overall quality and validity of data. To further advance the establishment of such controls, we designed and generated a new class of reference EVs expressing horseradish peroxidase (HRP) to facilitate simple and reliable EV tracing during isolation and standardization of EV purification and downstream analysis processes. HRP+ EVs can be quantified easily and with unmatched sensitivity either directly via measuring HRP activity or indirectly via immunodetection of HRP on the EV surface. We demonstrate that HRP+ EVs allow the reliable quantification of absolute EV numbers in biological or medical samples to normalize clinical specimens in liquid biopsies.
© 2025 Eximmium Biotechnologies GmbH. Journal of Extracellular Vesicles published by Wiley Periodicals LLC on behalf of International Society for Extracellular Vesicles.
Product Citations: 130
Development of Broadly Applicable Reference EVs Expressing Horseradish Peroxidase.
In Journal of Extracellular Vesicles on 1 August 2025 by Waas, D., Juraschitz, M., et al.
In The EMBO Journal on 1 May 2025 by Zheng, W., Pu, M., et al.
Lipid transfer proteins mediate the non-vesicular transport of lipids at membrane contact sites to regulate the lipid composition of organelle membranes. Despite significant recent advances in our understanding of the structural basis for lipid transfer, its functional regulation remains unclear. In this study, we report that S-palmitoylation modulates the cellular function of ATG2, a rod-like lipid transfer protein responsible for transporting phospholipids from the endoplasmic reticulum (ER) to phagophores during autophagosome formation. During starvation-induced autophagy, ATG2A undergoes depalmitoylation as the balance between ZDHHC11-mediated palmitoylation and APT1-mediated depalmitoylation. Inhibition of ATG2A depalmitoylation leads to impaired autophagosome formation and disrupted autophagic flux. Further, in cell and in vitro analyses demonstrate that S-palmitoylation at the C-terminus of ATG2A anchors the C-terminus to the ER. Depalmitoylation detaches the C-terminus from the ER membrane, enabling it to interact with phagophores and promoting their growth. These findings elucidate a S-palmitoylation-dependent regulatory mechanism of cellular ATG2, which may represent a broad regulatory strategy for lipid transport mediated by bridge-like transporters within cells.
© 2025. The Author(s).
-
Cell Biology
In Stem Cell Research & Therapy on 24 April 2025 by Ulpiano, C., Salvador, W., et al.
Mesenchymal-stromal-cell-derived extracellular vesicles (MSC-EVs) play a key role in the paracrine effects of MSC and have demonstrated therapeutic potential in various preclinical models. However, clinical translation is hindered by manufacturing practices relying on planar culture systems, fetal bovine serum (FBS)-supplemented media, and non-scalable, low-purity EV isolation methods that fail to meet dose and safety requirements, underscoring the need for innovative approaches. In this study, we developed a scalable platform to manufacture human MSC-EVs at clinically relevant numbers, integrating continuous collection of EV-enriched conditioned media (CM) using a stirred-tank reactor (STR) under xenogeneic-free conditions and a scalable downstream process.
Wharton's jelly-derived MSC (MSC(WJ)) were expanded using microcarriers in a controlled STR using human platelet lysate (hPL)-supplemented medium. Then, a 3-day EV production stage, featuring continuous harvesting of the CM, was established using a novel serum-/xeno(geneic)-free exosome depleted-hPL supplement. For the isolation of MSC-EVs, a scalable process was implemented by pairing tangential flow filtration and anion exchange chromatography. Isolated MSC-EVs were characterised using nanoparticle tracking analysis, protein and zeta potential quantification, western blot analysis of EV protein markers, transmission electron microscopy and uptake studies of fluorescently labelled-EVs.
The system sustained the efficient expansion of MSC(WJ), reaching a total of (6.03 ± 0.181) x 107 cells after 7 days, which corresponds to a 30.1 ± 0.740-fold expansion. Upon a 3-day continuous CM harvesting, a total of (2.13 ± 0.301) x 1012 EVs were isolated corresponding to a particle yield factor of (1.26 ± 0.186) x 104 EVs/cell/day. MSC-EVs presented high purity levels ((5.53 ± 1.55) x 109 particles/µg), a homogeneous small size distribution (mean diameter of 115 ± 4.88 nm), a surface charge of -23.4 ± 6.23 mV, positive detection of tetraspanins CD9 and CD63 and syntenin-1 and displayed a typical cup-shaped morphology. MSC-EVs were readily incorporated by endothelial cells and two human breast cancer cell lines.
Overall, the scalable and Good Manufacturing Practices (GMP)-compliant platform established herein enabled the reproducible manufacturing of MSC-EVs with high purity and generally accepted characteristics concerning size, protein markers, surface charge, morphology, and cellular internalization, validating its potential for future clinical applications.
© 2025. The Author(s).
-
WB
-
Homo sapiens (Human)
-
Stem Cells and Developmental Biology
In IScience on 21 March 2025 by Botelho, H. M., Lopes-Pacheco, M., et al.
Cystic fibrosis (CF) is a life-shortening disease affecting >160,000 individuals worldwide predominantly with respiratory symptoms. About 80% of individuals with CF have the p.Phe508del variant that causes the CF transmembrane conductance regulator (CFTR) protein to misfold and be targeted for premature degradation by the endoplasmic reticulum (ER) quality control (ERQC), thus preventing its plasma membrane (PM) traffic. Despite the recent approval of a "highly effective" drug rescuing p.Phe508del-CFTR, maximal lung function improvement is ∼14%. To identify global modulators of p.Phe508del traffic, we performed a high-content small interfering RNA (siRNA) microscopy-based screen of >9,000 genes and monitored p.Phe508del-CFTR PM rescue in human airway cells. This primary screen identified 227 p.Phe508del-CFTR traffic regulators, of which 35 could be validated by additional siRNAs. Subsequent mechanistic studies established GRK5 as a robust regulator whose inhibition rescues p.Phe508del-CFTR PM traffic and function in primary and immortalized cells, thus emerging as a novel potential drug target for CF.
© 2025 The Author(s).
Preprint on BioRxiv : the Preprint Server for Biology on 6 December 2024 by Rebendenne, A., Soulet, C., et al.
Like all viruses, SARS-CoV-2, the causative agent of COVID-19, relies on host cell resources to replicate. Our study reveals that, among these resources, SARS-CoV-2 hijacks the oxysterol-binding protein 1 (OSBP1) transporter to exploit the Golgi-bound phosphatidylinositol-4-phosphate (PI4P) pool. This leads to a depletion of Golgi-resident PI4P, triggering the activation of the ATM DNA Damage Response (DDR) kinase in the cytoplasm. As such, ATM, typically anchored to PI4P at the Golgi in an inactive state, undergoes auto-phosphorylation and cytoplasmic release upon SARS-CoV-2-induced PI4P depletion. Conversely, pharmacological inhibition of ATM auto-phosphorylation, which stabilizes its interaction with PI4P, significantly impairs SARS-CoV-2 replication. The requirement for PI4P and impact of ATM inhibition might be conserved across coronaviruses, as similar effects were observed with HCoV-229E. Finally, SARS-CoV-2-induced, cytoplasmic ATM pre-activation primes cells for an accelerated response to DNA damage, which might contribute to the severe outcomes of COVID-19 observed in cancer patients undergoing chemo- or radiotherapy. Therefore, this study uncovers a DNA damage-independent mode of ATM activation and highlights the potential of ATM inhibitors as therapeutic agents against COVID-19.
-
Cell Biology
-
COVID-19
-
Genetics
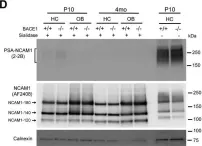
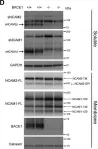
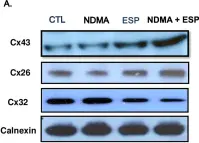
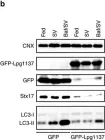
In Sci Adv on 23 June 2023 by Levy-Myers, R., Daudelin, D., et al.
Fig.1.D

-
WB
-
Collected and cropped from Sci Adv by CiteAb, provided under a CC-BY license
Image 1 of 32
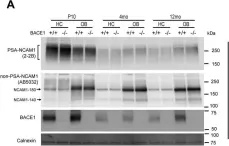
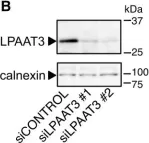
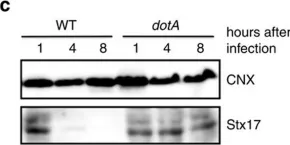
In PLoS Genet on 1 December 2021 by Albanese, M., Chen, Y. A., et al.
Fig.1.C

-
WB
-
Collected and cropped from PLoS Genet by CiteAb, provided under a CC-BY license
Image 1 of 32
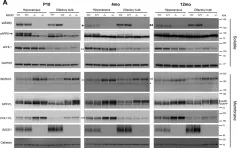
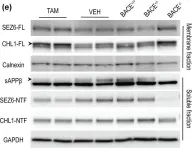
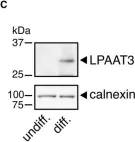
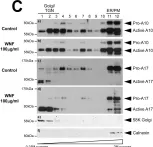
In Int J Mol Sci on 12 July 2021 by Ovejero, S., Soulet, C., et al.
Fig.1.C

-
WB
-
Homo sapiens (Human)
Collected and cropped from Int J Mol Sci by CiteAb, provided under a CC-BY license
Image 1 of 32
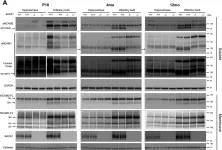
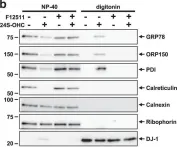
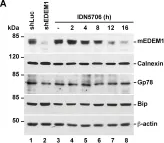
In J Biol Chem on 7 February 2021 by Kim, W., Watanabe, H., et al.
Fig.7.B

-
WB
-
Collected and cropped from J Biol Chem by CiteAb, provided under a CC-BY license
Image 1 of 32
In J Biol Chem on 7 February 2021 by Kim, W., Watanabe, H., et al.
Fig.7.D

-
WB
-
Collected and cropped from J Biol Chem by CiteAb, provided under a CC-BY license
Image 1 of 32
In J Biol Chem on 7 February 2021 by Kim, W., Watanabe, H., et al.
Fig.7.A

-
WB
-
Collected and cropped from J Biol Chem by CiteAb, provided under a CC-BY license
Image 1 of 32
In J Biol Chem on 7 February 2021 by Kim, W., Watanabe, H., et al.
Fig.6.A

-
WB
-
Collected and cropped from J Biol Chem by CiteAb, provided under a CC-BY license
Image 1 of 32
In J Biol Chem on 7 February 2021 by Kim, W., Watanabe, H., et al.
Fig.5.A

-
WB
-
Collected and cropped from J Biol Chem by CiteAb, provided under a CC-BY license
Image 1 of 32
In J Biol Chem on 7 February 2021 by Kim, W., Watanabe, H., et al.
Fig.1.D

-
WB
-
Collected and cropped from J Biol Chem by CiteAb, provided under a CC-BY license
Image 1 of 32
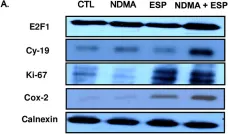
In Sci Rep on 27 December 2019 by Lombardo, S., Chiacchiaretta, M., et al.
Fig.5.E

-
WB
-
Mus musculus (House mouse)
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 32
In Cell Death Discov on 10 July 2019 by Urano, Y., Vo, D. H., et al.
Fig.4.B

-
WB
-
Homo sapiens (Human)
Collected and cropped from Cell Death Discov by CiteAb, provided under a CC-BY license
Image 1 of 32
In PLoS Negl Trop Dis on 1 April 2019 by Kim, E. M., Bae, Y. M., et al.
Fig.2.A

-
WB
-
Rattus norvegicus (Rat)
Collected and cropped from PLoS Negl Trop Dis by CiteAb, provided under a CC-BY license
Image 1 of 32
In PLoS Negl Trop Dis on 1 April 2019 by Kim, E. M., Bae, Y. M., et al.
Fig.3.A

-
WB
-
Rattus norvegicus (Rat)
Collected and cropped from PLoS Negl Trop Dis by CiteAb, provided under a CC-BY license
Image 1 of 32
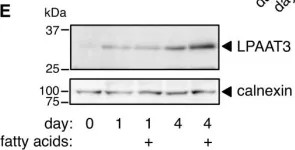
In J Lipid Res on 1 February 2018 by Valentine, W. J., Tokuoka, S. M., et al.
Fig.4.B

-
WB
-
Mus musculus (House mouse)
Collected and cropped from J Lipid Res by CiteAb, provided under a CC-BY license
Image 1 of 32
In J Lipid Res on 1 February 2018 by Valentine, W. J., Tokuoka, S. M., et al.
Fig.2.E

-
WB
-
Mus musculus (House mouse)
Collected and cropped from J Lipid Res by CiteAb, provided under a CC-BY license
Image 1 of 32
In J Lipid Res on 1 February 2018 by Valentine, W. J., Tokuoka, S. M., et al.
Fig.1.C

-
WB
-
Mus musculus (House mouse)
Collected and cropped from J Lipid Res by CiteAb, provided under a CC-BY license
Image 1 of 32
In Nat Commun on 1 November 2017 by Matsudaira, T., Mukai, K., et al.
Fig.1.D

-
WB
-
Homo sapiens (Human)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 32
In Nat Commun on 15 May 2017 by Arasaki, K., Mikami, Y., et al.
Fig.3.C

-
WB
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 32
In Nat Commun on 15 May 2017 by Arasaki, K., Mikami, Y., et al.
Fig.3.B

-
WB
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 32
In Nat Commun on 15 May 2017 by Arasaki, K., Mikami, Y., et al.
Fig.3.A

-
WB
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 32
In Nat Commun on 15 May 2017 by Arasaki, K., Mikami, Y., et al.
Fig.6.B

-
WB
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 32
In Nat Commun on 15 May 2017 by Arasaki, K., Mikami, Y., et al.
Fig.1.C

-
WB
-
Mus musculus (House mouse)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 32
In PLoS One on 21 January 2017 by Htoo, H. H., Limsuvan, S., et al.
Fig.5.C

-
WB
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 32
In PLoS One on 27 August 2015 by Cavieres, V. A., Gonzalez, A., et al.
Fig.1.A

-
WB
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 32
In Traffic on 1 April 2015 by Han, B., Tiwari, A., et al.
Fig.10.G

-
WB
-
Collected and cropped from Traffic by CiteAb, provided under a CC-BY license
Image 1 of 32