Disruptions of the eukaryotic plasma membrane due to chemical and mechanical challenges are frequent and detrimental, and thus need to be repaired to maintain proper cell function and avoid cell death. However, the cellular mechanisms involved in wound resealing and restoration of homeostasis are diverse and contended. Here, we show that clathrin-mediated endocytosis is induced at later stages of plasma membrane wound repair following the actual resealing of the wound. This compensatory endocytosis occurs near the wound, predominantly at sites of previous early endosome exocytosis which is required in the initial stage of membrane resealing, suggesting a spatio-temporal co-ordination of exo- and endocytosis during wound repair. Using cytoskeletal alterations and modulation of membrane tension and membrane area, we identify membrane tension as a major regulator of the wounding-associated exo- and endocytic events that mediate efficient wound repair. Thus, membrane tension changes are a universal trigger for plasma membrane wound repair modulating the exocytosis of early endosomes required for resealing and subsequent clathrin-mediated endocytosis acting at later stages to restore cell homeostasis and function.
Product Citations: 36
Membrane tension regulation is required for wound repair
Preprint on BioRxiv : the Preprint Server for Biology on 16 August 2024 by Raj, N., Weiss, M., et al.
-
Homo sapiens (Human)
In Scientific Reports on 14 February 2024 by Gupta, N., Waas, B., et al.
Homozygous Apolipoprotein L1 (APOL1) variants G1 and G2 cause APOL1-mediated kidney disease, purportedly acting as surface cation channels in podocytes. APOL1-G0 exhibits various single nucleotide polymorphisms, most commonly haplotype E150K, M228I and R255K ("KIK"; the Reference Sequence is "EMR"), whereas variants G1 and G2 are mostly found in a single "African" haplotype background ("EIK"). Several labs reported cytotoxicity with risk variants G1 and G2 in KIK or EIK background haplotypes, but used HEK-293 cells and did not verify equal surface expression. To see if haplotype matters in a more relevant cell type, we induced APOL1-G0, G1 and G2 EIK, KIK and EMR at comparable surface levels in immortalized podocytes. G1 and G2 risk variants (but not G0) caused dose-dependent podocyte death within 48h only in their native African EIK haplotype and correlated with K+ conductance (thallium FLIPR). We ruled out differences in localization and trafficking, except for possibly greater surface clustering of cytotoxic haplotypes. APOL1 surface expression was required, since Brefeldin A rescued cytotoxicity; and cytoplasmic isoforms vB3 and vC were not cytotoxic. Thus, APOL1-EIK risk variants kill podocytes in a dose and haplotype-dependent manner (as in HEK-293 cells), whereas unlike in HEK-293 cells the KIK risk variants did not.
© 2024. The Author(s).
In Journal of Extracellular Vesicles on 1 December 2023 by Barreca, V., Boussadia, Z., et al.
Exosomes are among the most puzzling vehicles of intercellular communication, but several crucial aspects of their biogenesis remain elusive, primarily due to the difficulty in purifying vesicles with similar sizes and densities. Here we report an effective methodology for labelling small extracellular vesicles (sEV) using Bodipy FL C16, a fluorescent palmitic acid analogue. In this study, we present compelling evidence that the fluorescent sEV population derived from Bodipy C16-labelled cells represents a discrete subpopulation of small exosomes following an intracellular pathway. Rapid cellular uptake and metabolism of Bodipy C16 resulted in the incorporation of fluorescent phospholipids into intracellular organelles specifically excluding the plasma membrane and ultimately becoming part of the exosomal membrane. Importantly, our fluorescence labelling method facilitated accurate quantification and characterization of exosomes, overcoming the limitations of nonspecific dye incorporation into heterogeneous vesicle populations. The characterization of Bodipy-labelled exosomes reveals their enrichment in tetraspanin markers, particularly CD63 and CD81, and in minor proportion CD9. Moreover, we employed nanoFACS sorting and electron microscopy to confirm the exosomal nature of Bodipy-labelled vesicles. This innovative metabolic labelling approach, based on the fate of a fatty acid, offers new avenues for investigating exosome biogenesis and functional properties in various physiological and pathological contexts.
© 2023 The Authors. Journal of Extracellular Vesicles published by Wiley Periodicals LLC on behalf of International Society for Extracellular Vesicles.
-
Homo sapiens (Human)
-
Biochemistry and Molecular biology
-
Cell Biology
ATG9 vesicles comprise the seed membrane of mammalian autophagosomes.
In The Journal of Cell Biology on 3 July 2023 by Olivas, T. J., Wu, Y., et al.
As the autophagosome forms, its membrane surface area expands rapidly, while its volume is kept low. Protein-mediated transfer of lipids from another organelle to the autophagosome likely drives this expansion, but as these lipids are only introduced into the cytoplasmic-facing leaflet of the organelle, full membrane growth also requires lipid scramblase activity. ATG9 harbors scramblase activity and is essential to autophagosome formation; however, whether ATG9 is integrated into mammalian autophagosomes remains unclear. Here we show that in the absence of lipid transport, ATG9 vesicles are already competent to collect proteins found on mature autophagosomes, including LC3-II. Further, we use styrene-maleic acid lipid particles to reveal the nanoscale organization of protein on LC3-II membranes; ATG9 and LC3-II are each fully integrated into expanding autophagosomes. The ratios of these two proteins at different stages of maturation demonstrate that ATG9 proteins are not continuously integrated, but rather are present on the seed vesicles only and become diluted in the expanding autophagosome membrane.
© 2023 Olivas et al.
-
Cell Biology
-
Plant Science
Early Endosomes Act as Local Exocytosis Hubs to Repair Endothelial Membrane Damage.
In Advanced Science (Weinheim, Baden-Wurttemberg, Germany) on 1 May 2023 by Raj, N., Greune, L., et al.
The plasma membrane of a cell is subject to stresses causing ruptures that must be repaired immediately to preserve membrane integrity and ensure cell survival. Yet, the spatio-temporal membrane dynamics at the wound site and the source of the membrane required for wound repair are poorly understood. Here, it is shown that early endosomes, previously only known to function in the uptake of extracellular material and its endocytic transport, are involved in plasma membrane repair in human endothelial cells. Using live-cell imaging and correlative light and electron microscopy, it is demonstrated that membrane injury triggers a previously unknown exocytosis of early endosomes that is induced by Ca2+ entering through the wound. This exocytosis is restricted to the vicinity of the wound site and mediated by the endosomal soluble N-ethylmaleimide sensitive factor attachment protein receptor (SNARE) VAMP2, which is crucial for efficient membrane repair. Thus, the newly identified Ca2+ -evoked and localized exocytosis of early endosomes supplies the membrane material required for rapid resealing of a damaged plasma membrane, thereby providing the first line of defense against damage in mechanically challenged endothelial cells.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
-
Cell Biology
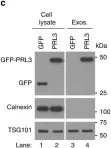
In Nat Commun on 6 June 2019 by Thura, M., Al-Aidaroos, A. Q., et al.
Fig.3.C

-
WB
-
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 5
In J Exp Clin Cancer Res on 5 October 2018 by Boussadia, Z., Lamberti, J., et al.
Fig.3.C,D

-
WB
-
Homo sapiens (Human)
Collected and cropped from J Exp Clin Cancer Res by CiteAb, provided under a CC-BY license
Image 1 of 5
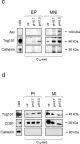
In J Exp Clin Cancer Res on 5 October 2018 by Boussadia, Z., Lamberti, J., et al.
Fig.3.C

-
WB
-
Collected and cropped from J Exp Clin Cancer Res by CiteAb, provided under a CC-BY license
Image 1 of 5
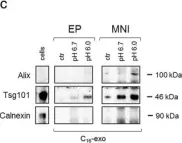
In PLoS One on 10 January 2013 by Beilstein, F., Bouchoux, J., et al.
Fig.3.A

-
WB
-
Homo sapiens (Human)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 5
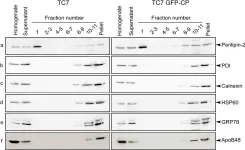
In PLoS One on 23 August 2011 by Scharadin, T. M., Jiang, H., et al.
Fig.7.A

-
WB
-
Homo sapiens (Human)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 5