Lisavanbulin is a prodrug of the microtubule-targeting agent avanbulin. Both avanbulin and lisavanbulin have demonstrated significant antitumor activity in several preclinical tumor models including glioblastoma. Previous human studies demonstrated that 48-h infusions of intravenous lisavanbulin were well tolerated with preliminary activity in recurrent glioblastoma. The current phase 1/2a study evaluates the safety and tolerability of once-daily oral lisavanbulin in patients with solid tumors or recurrent glioblastoma or high-grade glioma. Lisavanbulin is associated with profound, durable responses in a subset of patients with recurrent refractory grade 4 astrocytoma or glioblastoma. We present here the clinical and translational results from this trial, including a description of a response-predictive molecular signature that warrants further exploration in these tumor types of significant unmet need. The study is registered at ClinicalTrials.gov (NCT02490800).
Copyright © 2025 The Author(s). Published by Elsevier Inc. All rights reserved.
Product Citations: 46
In Cell Reports Medicine on 17 June 2025 by Lopez, J. S., Haefliger, S., et al.
-
Cancer Research
Functional genetics reveals modulators of antimicrotubule drug sensitivity.
In The Journal of Cell Biology on 3 February 2025 by Su, K. C., Radul, E., et al.
Microtubules play essential roles in diverse cellular processes and are important pharmacological targets for treating human disease. Here, we sought to identify cellular factors that modulate the sensitivity of cells to antimicrotubule drugs. We conducted a genome-wide CRISPR/Cas9-based functional genetics screen in human cells treated with the microtubule-destabilizing drug nocodazole or the microtubule-stabilizing drug paclitaxel. We further conducted a focused secondary screen to test drug sensitivity for ∼1,400 gene targets across two distinct human cell lines and to additionally test sensitivity to the KIF11 inhibitor, STLC. These screens defined gene targets whose loss enhances or suppresses sensitivity to antimicrotubule drugs. In addition to gene targets whose loss sensitized cells to multiple compounds, we observed cases of differential sensitivity to specific compounds and differing requirements between cell lines. Our downstream molecular analysis further revealed additional roles for established microtubule-associated proteins and identified new players in microtubule function.
© 2024 Su et al.
-
Cell Biology
-
Genetics
Preprint on BioRxiv : the Preprint Server for Biology on 13 January 2025 by Shao, J., Zhang, R., et al.
Microtubules are primarily studied for the interactions of proteins that bind to their outer surfaces and ends, while the regulatory mechanisms within the microtubule lumen, particularly in singlet microtubules critical for essential cellular processes, remain largely unexplored. Our study provides the first systematic identification of key regulatory proteins within the single microtubule lumen. Using proximity-dependent biotin identification (Bio-ID) coupled with mass spectrometry, we identified candidate microtubule inner proteins (MIPs), including Jupiter microtubule-associated homolog 2 (JPT2). JPT2 binds directly to microtubules and specifically localizes within the lumen, where it modulates the luminal environment by inhibiting acetylase MEC17 and independently affects the binding and efficacy of Paclitaxel. Furthermore, our screening identified additional MIPs that influence cellular sensitivity to Paclitaxel, indicating a link between luminal regulation and drug responsiveness. These discoveries reveal JPT2’s critical role in singlet microtubule regulation and suggest new therapeutic targets for enhancing cancer drug sensitivity.
-
IF
-
Homo sapiens (Human)
α-tubulin detyrosination fine-tunes kinetochore-microtubule attachments.
In Nature Communications on 9 November 2024 by Girão, H., Macário-Monteiro, J., et al.
Post-translational cycles of α-tubulin detyrosination and tyrosination generate microtubule diversity, the cellular functions of which remain largely unknown. Here we show that α-tubulin detyrosination regulates kinetochore-microtubule attachments to ensure normal chromosome oscillations and timely anaphase onset during mitosis. Remarkably, detyrosinated α-tubulin levels near kinetochore microtubule plus-ends depend on the direction of chromosome motion during metaphase. Proteomic analyses unveil that the KNL-1/MIS12/NDC80 (KMN) network that forms the core microtubule-binding site at kinetochores and the microtubule-rescue protein CLASP2 are enriched on tyrosinated and detyrosinated microtubules during mitosis, respectively. α-tubulin detyrosination enhances CLASP2 binding and NDC80 complex diffusion along the microtubule lattice in vitro. Rescue experiments overexpressing NDC80, including variants with slower microtubule diffusion, suggest a functional interplay with α-tubulin detyrosination for the establishment of a labile kinetochore-microtubule interface. These results offer a mechanistic explanation for how different detyrosinated α-tubulin levels near kinetochore microtubule plus-ends fine-tune load-bearing attachments to both growing and shrinking microtubules.
© 2024. The Author(s).
-
Cell Biology
Systems mapping of bidirectional endosomal transport through the crowded cell.
In Current Biology : CB on 7 October 2024 by Jongsma, M. L. M., Bakker, N., et al.
Kinesin and dynein-dynactin motors move endosomes and other vesicles bidirectionally along microtubules, a process mainly studied under in vitro conditions. Here, we provide a physiological bidirectional transport model following color-coded, endogenously tagged transport-related proteins as they move through a crowded cellular environment. Late endosomes (LEs) surf bidirectionally on Protrudin-enriched endoplasmic reticulum (ER) membrane contact sites, while hopping and gliding along microtubules and bypassing cellular obstacles, such as mitochondria. During bidirectional transport, late endosomes do not switch between opposing Rab7 GTPase effectors, RILP and FYCO1, or their associated dynein and KIF5B motor proteins, respectively. In the endogenous setting, far fewer motors associate with endosomal membranes relative to effectors, implying coordination of transport with other aspects of endosome physiology through GTPase-regulated mechanisms. We find that directionality of transport is provided in part by various microtubule-associated proteins (MAPs), including MID1, EB1, and CEP169, which recruit Lis1-activated dynein motors to microtubule plus ends for transport of early and late endosomal populations. At these microtubule plus ends, activated dynein motors encounter the dynactin subunit p150glued and become competent for endosomal capture and minus-end movement in collaboration with membrane-associated Rab7-RILP. We show that endosomes surf over the ER through the crowded cell and move bidirectionally under the control of MAPs for motor activation and through motor replacement and capture by endosomal anchors.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
-
Homo sapiens (Human)
-
Cell Biology
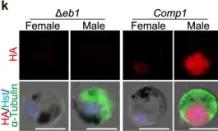
In Nat Commun on 19 May 2023 by Yang, S., Cai, M., et al.
Fig.1.K
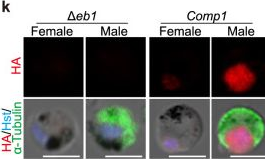
-
ICC-IF
-
Collected and cropped from Nature Communications by CiteAb, provided under a CC-BY license
Image 1 of 1