Metabolic deregulation is a major hallmark of cancer, therefore, interventions that modify tumor nutrient availability are considered attractive adjuvants for improving clinical outcomes for cancer patients. Much work remains, however, in clarifying how the nutritional status of each patient can affect the metabolic vulnerability of drugs and inform individual medication guidelines. Working toward the goal of oncometabolic precision medicine, we introduce CM-SLP (cancer metabolism-based synthetic lethality platform), a high-throughput screening platform that explores the metabolic vulnerability of non-oncology drugs induced by altered nutrient availability and predicts the potential synthetic lethal interactions with either hyper- or hypo-nutrient conditions. We present promising CM-SLP candidates, such as propafenone and biguanides, as representative non-oncology drugs that cooperatively enhance cytotoxicity via dysregulated metabolic pathways. Furthermore, identifying mTOR and Hippo pathways as mediators of combined propafenone/hypoglycemia or biguanides/hypoglycemia treatments, respectively, we were able to circumvent the need for dietary interventions by administering the mTOR or TEAD inhibitors to induce energy stress and cancer cell death. Together, CM-SLP represents a critical step toward integrating metabolic profiling into precision oncology, offering novel therapeutic avenues tailored to individual patient needs.
Product Citations: 37
Nutrient availability dictates cancer metabolism-based therapeutic responses of non-oncology drugs
Preprint on BioRxiv : the Preprint Server for Biology on 5 January 2025 by Pyun, W. Y., Park, J. H., et al.
-
Biochemistry and Molecular biology
-
Cancer Research
-
Cell Biology
Focal adhesions contain three specialized actin nanoscale layers.
In Nature Communications on 21 March 2024 by Kumari, R., Ven, K., et al.
Focal adhesions (FAs) connect inner workings of cell to the extracellular matrix to control cell adhesion, migration and mechanosensing. Previous studies demonstrated that FAs contain three vertical layers, which connect extracellular matrix to the cytoskeleton. By using super-resolution iPALM microscopy, we identify two additional nanoscale layers within FAs, specified by actin filaments bound to tropomyosin isoforms Tpm1.6 and Tpm3.2. The Tpm1.6-actin filaments, beneath the previously identified α-actinin cross-linked actin filaments, appear critical for adhesion maturation and controlled cell motility, whereas the adjacent Tpm3.2-actin filament layer beneath seems to facilitate adhesion disassembly. Mechanistically, Tpm3.2 stabilizes ACF-7/MACF1 and KANK-family proteins at adhesions, and hence targets microtubule plus-ends to FAs to catalyse their disassembly. Tpm3.2 depletion leads to disorganized microtubule network, abnormally stable FAs, and defects in tail retraction during migration. Thus, FAs are composed of distinct actin filament layers, and each may have specific roles in coupling adhesions to the cytoskeleton, or in controlling adhesion dynamics.
© 2024. The Author(s).
-
Cell Biology
3D matrix adhesion feedback controls nuclear force coupling to drive invasive cell migration.
In Cell Reports on 26 December 2023 by Newman, D., Young, L. E., et al.
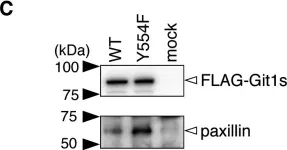
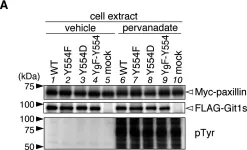
Cell invasion is a multi-step process, initiated by the acquisition of a migratory phenotype and the ability to move through complex 3D extracellular environments. We determine the composition of cell-matrix adhesion complexes of invasive breast cancer cells in 3D matrices and identify an interaction complex required for invasive migration. βPix and myosin18A (Myo18A) drive polarized recruitment of non-muscle myosin 2A (NM2A) to adhesion complexes at the tips of protrusions. Actomyosin force engagement then displaces the Git1-βPix complex from paxillin, establishing a feedback loop for adhesion maturation. We observe active force transmission to the nucleus during invasive migration that is needed to pull the nucleus forward. The recruitment of NM2A to adhesions creates a non-muscle myosin isoform gradient, which extends from the protrusion to the nucleus. We postulate that this gradient facilitates coupling of cell-matrix interactions at the protrusive cell front with nuclear movement, enabling effective invasive migration and front-rear cell polarity.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
The weakness of senescent dermal fibroblasts.
In Proceedings of the National Academy of Sciences of the United States of America on 22 August 2023 by Rebehn, L., Khalaji, S., et al.
Skin is the largest human organ with easily noticeable biophysical manifestations of aging. As human tissues age, there is chronological accumulation of biophysical changes due to internal and environmental factors. Skin aging leads to decreased elasticity and the loss of dermal matrix integrity via degradation. The mechanical properties of the dermal matrix are maintained by fibroblasts, which undergo replicative aging and may reach senescence. While the secretory phenotype of senescent fibroblasts is well studied, little is known about changes in the fibroblasts biophysical phenotype. Therefore, we compare biophysical properties of young versus proliferatively aged primary fibroblasts via fluorescence and traction force microscopy, single-cell atomic force spectroscopy, microfluidics, and microrheology of the cytoskeleton. Results show senescent fibroblasts have decreased cytoskeletal tension and myosin II regulatory light chain phosphorylation, in addition to significant loss of traction force. The alteration of cellular forces is harmful to extracellular matrix homeostasis, while decreased cytoskeletal tension can amplify epigenetic changes involved in senescence. Further exploration and detection of these mechanical phenomena provide possibilities for previously unexplored pharmaceutical targets against aging.
-
Homo sapiens (Human)
Preprint on BioRxiv : the Preprint Server for Biology on 20 August 2023 by Chantachotikul, P., Liu, S., et al.
Aging proceeds with accumulation of senescent cells in multiple organs. Senescent cells become large in size compared to young cells, which promotes further senescence and age-related diseases. Currently, the molecular mechanism behind the maintenance of such huge cell architecture undergoing senescence remains poorly understood. Here we focus on reorganization of actin stress fibers induced upon replicative senescence of human fibroblasts, typically used as a senescent cell model. We identified, together with our previous proteomic study, that AP2A1 (alpha 1 adaptin subunit of the adaptor protein 2) is upregulated in senescent cells along the length of stress fibers, which are enlarged following the increase in the whole cell size. We then revealed that knockdown of AP2A1 in senescent cells suppresses key senescence-associated phenotypes, which include decreased cell area and lowered expression of major senescence markers. Meanwhile, AP2A1 overexpression in young cells induced the opposite effects that rather advance senescence, suggesting that AP2A1 may be used as a senescence marker. We found that AP2A1 is colocalized with integrin β1, and both of them move linearly along stress fibers. We further observed that focal adhesions are enlarged in senescent cells to reinforce cell adhesions to the substrate. These results suggest that senescent cells maintain their large size by strengthening the anchorage to the substrate by supplying integrin β1 via translocation along stress fibers. This mechanism may work efficiently in senescent cells, compared with a case relying on random diffusion of integrin β1, given the enlarged cell size and resulting increase in travel time and distance for endocytosed vesicle transportation.
-
Homo sapiens (Human)
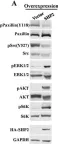
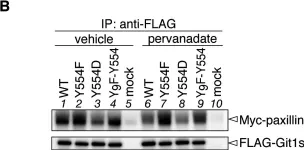
In Oncotarget on 8 November 2016 by Zhang, R. Y., Yu, Z. H., et al.
Fig.3.A

-
WB
-
Collected and cropped from Oncotarget by CiteAb, provided under a CC-BY license
Image 1 of 10
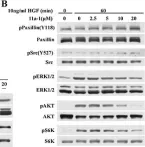
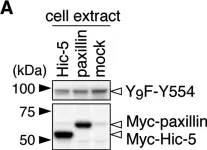
In Oncotarget on 8 November 2016 by Zhang, R. Y., Yu, Z. H., et al.
Fig.5.B

-
WB
-
Collected and cropped from Oncotarget by CiteAb, provided under a CC-BY license
Image 1 of 10
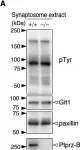
In PLoS One on 6 March 2015 by Fujikawa, A., Matsumoto, M., et al.
Fig.1.C

-
WB
-
Homo sapiens (Human)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 10
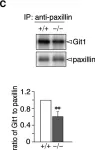
In PLoS One on 6 March 2015 by Fujikawa, A., Matsumoto, M., et al.
Fig.3.A

-
WB
-
Homo sapiens (Human)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 10
In PLoS One on 6 March 2015 by Fujikawa, A., Matsumoto, M., et al.
Fig.3.B

-
ChIP
-
Homo sapiens (Human)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 10
In PLoS One on 6 March 2015 by Fujikawa, A., Matsumoto, M., et al.
Fig.4.A

-
WB
-
Homo sapiens (Human)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 10
In PLoS One on 6 March 2015 by Fujikawa, A., Matsumoto, M., et al.
Fig.5.A

-
WB
-
Mus musculus (House mouse)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 10
In PLoS One on 6 March 2015 by Fujikawa, A., Matsumoto, M., et al.
Fig.5.C

-
WB
-
Mus musculus (House mouse)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 10
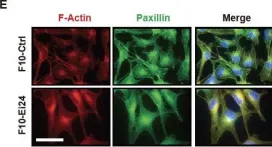
In Oncotarget on 1 December 2013 by Choi, J. M., Devkota, S., et al.
Fig.1.E

-
ICC-IF
-
Collected and cropped from Oncotarget by CiteAb, provided under a CC-BY license
Image 1 of 10
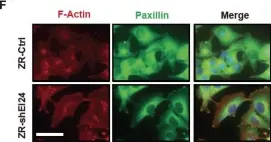
In Oncotarget on 1 December 2013 by Choi, J. M., Devkota, S., et al.
Fig.1.F

-
ICC-IF
-
Collected and cropped from Oncotarget by CiteAb, provided under a CC-BY license
Image 1 of 10