Focal adhesions serve as structural and signaling hubs, facilitating bidirectional communication at the cell-extracellular matrix interface. Paxillin and the related Hic-5 (TGFβ1i1) are adaptor/scaffold proteins that recruit numerous structural and regulatory proteins to focal adhesions, where they perform both overlapping and discrete functions. In this study, paxillin and Hic-5 were expressed in U2OS osteosarcoma cells as biotin ligase (BioID2) fusion proteins and used as bait proteins for proximity-dependent biotinylation in order to directly compare their respective interactomes. The fusion proteins localized to both focal adhesions and the centrosome, resulting in biotinylation of components of each of these structures. Biotinylated proteins were purified and analyzed by mass spectrometry. The list of proximity interactors for paxillin and Hic-5 comprised numerous shared core focal adhesion proteins that likely contribute to their similar functions in cell adhesion and migration, as well as proteins unique to paxillin and Hic-5 that have been previously localized to focal adhesions, the centrosome, or the nucleus. Western blotting confirmed biotinylation and enrichment of FAK and vinculin, known interactors of Hic-5 and paxillin, as well as several potentially unique proximity interactors of Hic-5 and paxillin, including septin 7 and ponsin, respectively. Further investigation into the functional relationship between the unique interactors and Hic-5 or paxillin may yield novel insights into their distinct roles in cell migration.
© 2024 Wiley Periodicals LLC.
Product Citations: 22
A comparative analysis of paxillin and Hic-5 proximity interactomes.
In Cytoskeleton (Hoboken, N.J.) on 1 January 2025 by Brock, K., Alpha, K. M., et al.
-
Homo sapiens (Human)
In Advanced Science (Weinheim, Baden-Wurttemberg, Germany) on 1 February 2024 by Zhang, C., Wang, Y., et al.
Cells constantly sense and respond to not only biochemical but also biomechanical changes in their microenvironment, demanding for dynamic metabolic adaptation. ECM stiffening is a hallmark of cancer aggressiveness, while survival under substrate detachment also associates with poor prognosis. Mechanisms underlying this, non-linear mechano-response of tumor cells may reveal potential double-hit targets for cancers. Here, an integrin-GSK3β-FTO-mTOR axis is reported, that can integrate stiffness sensing to ensure both the growth advantage endowed by rigid substrate and cell death resistance under matrix detachment. It is demonstrated that substrate stiffening can activate mTORC1 and elevate mTOR level through integrins and GSK3β-FTO mediated mRNA m6 A modification, promoting anabolic metabolism. Inhibition of this axis upon ECM detachment enhances autophagy, which in turn conveys resilience of tumor cells to anoikis, as it is demonstrated in human breast ductal carcinoma in situ (DCIS) and mice malignant ascites. Collectively, these results highlight the biphasic mechano-regulation of cellular metabolism, with implications in tumor growth under stiffened conditions such as fibrosis, as well as in anoikis-resistance during cancer metastasis.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
In Cell Communication and Signaling : CCS on 22 January 2024 by Zhang, Y., Li, N., et al.
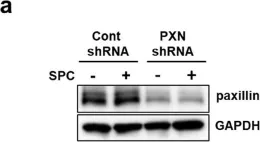
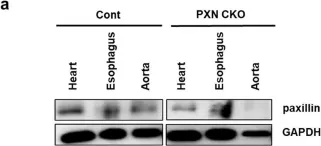
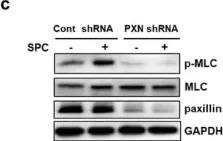
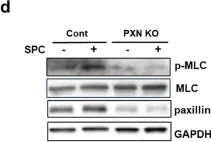
The Ca2+-independent contraction of vascular smooth muscle is a leading cause of cardiovascular and cerebrovascular spasms. In the previous study, we demonstrated the involvement of Src family protein tyrosine kinase Fyn and Rho-kinase in the sphingosylphosphorylcholine (SPC)-induced abnormal and Ca2+-independent contraction of vascular smooth muscle, but the specific mechanism has not been completely clarified.
Paxillin knockdown human coronary artery smooth muscle cells (CASMCs) and smooth muscle-specific paxillin knockout mice were generated by using paxillin shRNA and the tamoxifen-inducible Cre-LoxP system, respectively. CASMCs contraction was observed by time-lapse recording. The vessel contractility was measured by using a myography assay. Fyn, Rho-kinase, and myosin light chain activation were assessed by immunoprecipitation and western blotting. The paxillin expression and actin stress fibers were visualized by histological analysis and immunofluorescent staining.
The SPC-induced abnormal contraction was inhibited in paxillin knockdown CASMCs and arteries of paxillin knockout mice, indicating that paxillin is involved in this abnormal contraction. Further study showed that paxillin knockdown inhibited the SPC-induced Rho-kinase activation without affecting Fyn activation. In addition, paxillin knockdown significantly inhibited the SPC-induced actin stress fiber formation and myosin light chain phosphorylation. These results suggest that paxillin, as an upstream molecule of Rho-kinase, is involved in the SPC-induced abnormal contraction of vascular smooth muscle.
The present study demonstrated that paxillin participates in the SPC-induced abnormal vascular smooth muscle contraction by regulating Rho-kinase activation. Video Abstract.
© 2024. The Author(s).
-
WB
-
Endocrinology and Physiology
In Developmental Cell on 3 May 2021 by Wang, Y., Zhang, C., et al.
Cells sense and respond to extracellular mechanical cues through cell-matrix adhesions. Interestingly, the maturation of focal adhesions (FAs) is reciprocally force dependent. How biomechanical cues dictate the status of cell motility and how FAs spatial temporally coordinate force sensing and self-organization remain enigmatic. Here, we identify that LIMD1, a member of the LIM domain scaffolding proteins, undergoes force-sensitive condensation at the FAs. We also unveil that the multivalent interactions of LIMD1 intrinsically disordered region (IDR) and the LIM domains concertedly drive this phase transition under the regulation of phosphorylation. Intriguingly, formation of condensed LIMD1 protein compartments is sufficient to specifically enrich and localize late FA proteins. We further discover that LIMD1 regulates cell spreading, maintains FA dynamics and cellular contractility, and is critical for durotaxis-the ability of cells to crawl along gradients of substrate stiffness. Our results suggest a model that recruitment of LIMD1 to the FAs, via mechanical force triggered inter-molecular interaction, serves as a phase separation hub to assemble and organize matured FAs, thus allowing for efficient mechano-transduction and cell migration.
Copyright © 2021 Elsevier Inc. All rights reserved.
-
Stem Cells and Developmental Biology
In eLife on 15 February 2021 by Yang, H., Lin, L., et al.
Communications between actin filaments and integrin-mediated focal adhesion (FA) are crucial for cell adhesion and migration. As a core platform to organize FA proteins, the tripartite ILK/PINCH/Parvin (IPP) complex interacts with actin filaments to regulate the cytoskeleton-FA crosstalk. Rsu1, a Ras suppressor, is enriched in FA through PINCH1 and plays important roles in regulating F-actin structures. Here, we solved crystal structures of the Rsu1/PINCH1 complex, in which the leucine-rich-repeats of Rsu1 form a solenoid structure to tightly associate with the C-terminal region of PINCH1. Further structural analysis uncovered that the interaction between Rsu1 and PINCH1 blocks the IPP-mediated F-actin bundling by disrupting the binding of PINCH1 to actin. Consistently, overexpressing Rsu1 in HeLa cells impairs stress fiber formation and cell spreading. Together, our findings demonstrated that Rsu1 is critical for tuning the communication between F-actin and FA by interacting with the IPP complex and negatively modulating the F-actin bundling.
© 2021, Yang et al.
-
IF
-
Cell Biology
In Cell Commun Signal on 22 January 2024 by Zhang, Y., Li, N., et al.
Fig.1.A

-
WB
-
Collected and cropped from Cell Commun Signal by CiteAb, provided under a CC-BY license
Image 1 of 5
In Cell Commun Signal on 22 January 2024 by Zhang, Y., Li, N., et al.
Fig.2.A

-
WB
-
Collected and cropped from Cell Commun Signal by CiteAb, provided under a CC-BY license
Image 1 of 5
In Cell Commun Signal on 22 January 2024 by Zhang, Y., Li, N., et al.
Fig.6.C

-
WB
-
Collected and cropped from Cell Commun Signal by CiteAb, provided under a CC-BY license
Image 1 of 5
In Cell Commun Signal on 22 January 2024 by Zhang, Y., Li, N., et al.
Fig.6.D

-
WB
-
Collected and cropped from Cell Commun Signal by CiteAb, provided under a CC-BY license
Image 1 of 5
In Sci Rep on 17 August 2018 by Png, E., Hou, A., et al.
Fig.4.D

-
WB
-
Collected and cropped from Sci Rep by CiteAb, provided under a CC-BY license
Image 1 of 5