Loss-of-function mutations in AKAP11 (a protein kinase A (PKA)-binding protein) greatly increase the risk of bipolar disorder and schizophrenia. To determine the neurobiological functions of AKAP11, we conduct multi-omic and neurobiological analyses of Akap11 mutant mouse brains. We find that AKAP11 is a key regulator of PKA proteostasis in the brain whose loss leads to dramatically increased levels of PKA subunits and phosphorylated PKA substrates, especially in synapses. Akap11 mutant mice show extensive transcriptomic changes throughout the brain, including prominent decreases in synapse-related genes sets. Gene expression is highly impacted in spiny projection neurons of the striatum, a brain region implicated in motivation, cognition and psychotic disorders. Real-time measurements of PKA activity reveal elevated basal PKA activity in the striatum of Akap11-/- mice, with exaggerated additional response to dopamine receptor antagonists. Behaviorally, Akap11 mutant mice show abnormally prolonged locomotor response to amphetamine, deficits in associative learning and contextual discrimination, as well as depression-like behaviors. Our study connects molecular changes to circuit dysfunction and behavioral disturbance in a genetically valid animal model of psychotic disorder.
© 2025. The Author(s).
Product Citations: 72
In Nature Communications on 28 November 2025 by Song, B. J., Ge, Y., et al.
-
Genetics
BH4 Oxidation-Derived H2O2 Activates ERK1/2 Signaling via B-Raf in Rat Dorsal Root Ganglion Neurons.
In Journal of Neurochemistry on 1 November 2025 by Mohammadi, M., Siobal, M., et al.
Elevated Tetrahydrobiopterin (BH4) levels are linked to various pain conditions. Pharmacological or genetic reduction of BH4 levels has analgesic effects in rodent models of neuropathic and inflammatory pain, but also affects neurological and cardiovascular functions. Little is known about the downstream mechanisms of BH4 that could be targeted to attenuate BH4-induced pain hypersensitivity without lowering BH4 levels. In this study, we exposed ex vivo-cultured rat dorsal root ganglion (DRG) neurons to BH4 and analyzed the activity of the sensory neuron-sensitizing kinase ERK1/2 via high-content imaging. We show that BH4 exposure leads to increased pERK1/2 levels in a dose- and time-dependent manner. Interestingly, we found that H2O2, as a by-product of BH4 oxidation and not BH4 itself, induces increased pERK1/2 levels via MEK1/2 and B-Raf (but not A-Raf or C-Raf) and that this can be blocked by pharmacological interference. In conclusion, elevated BH4 levels, as observed in various pain conditions, may drive sensory neuron sensitization via oxidation-derived H2O2 and the B-Raf-MEK1/2-ERK1/2 axis, which presents a novel pathway that could be targeted to attenuate BH4-induced pain hypersensitivity without the necessity to reduce BH4 levels.
© 2025 The Author(s). Journal of Neurochemistry published by John Wiley & Sons Ltd on behalf of International Society for Neurochemistry.
-
Neuroscience
-
Plant Science
Afadin-deficient retinas exhibit severe neuronal lamination defects but preserve visual functions
Preprint on BioRxiv : the Preprint Server for Biology on 25 December 2024 by Ueno, A., Sakuta, K., et al.
ABSTRACT Neural lamination is a common feature of the central nervous system (CNS), with several subcellular structures, such as adherens junctions (AJs), playing a role in this process. The retina is also heavily laminated, but it remains unclear how laminar formation impacts retinal cell morphology, synapse integrity, and overall retinal function. In this study, we demonstrate that the loss of afadin, a key component of AJs, leads to significant pathological changes. These include the disruption of outer retinal lamination and a notable decrease as well as mislocalization of photoreceptors, their outer segments, and photoreceptor synapses. Interestingly, despite these severe impairments, we recorded small local field potentials, including the a- and b-waves. We also classified ganglion cells into ON, ON-OFF, and OFF types based on their firing patterns in response to light stimuli. Additionally, we successfully characterized the receptive fields of certain retinal ganglion cells. Overall, these findings provide the first evidence that retinal circuit function can be partially preserved even when there are significant disruptions in retinal lamination and photoreceptor synapses. Our results indicate that retinas with severely altered morphology still retain some capacity to process light stimuli.
-
IHC
-
Mus musculus (House mouse)
Necl-1/CADM3 regulates cone synapse formation in the mouse retina.
In IScience on 19 April 2024 by Kawashima, R., Matsushita, K., et al.
In vertebrates, retinal neural circuitry for visual perception is organized in specific layers. The outer plexiform layer is the first synaptic region in the visual pathway, where photoreceptor synaptic terminals connect with bipolar and horizontal cell processes. However, molecular mechanisms underlying cone synapse formation to mediate OFF pathways remain unknown. This study reveals that Necl-1/CADM3 is localized at S- and S/M-opsin-containing cones and dendrites of type 4 OFF cone bipolar cells (CBCs). In Necl-1-/- mouse retina, synapses between cones and type 4 OFF CBCs were dislocated, horizontal cell distribution became abnormal, and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors were dislocated. Necl-1-/- mice exhibited aberrant short-wavelength-light-elicited signal transmission from cones to OFF CBCs, which was rescued by AMPA receptor potentiator. Additionally, Necl-1-/- mice showed impaired optokinetic responses. These findings suggest that Necl-1 regulates cone synapse formation to mediate OFF cone pathways elicited by short-wavelength light in mouse retina.
© 2024 The Authors.
-
Neuroscience
Endosome positioning coordinates spatially selective GPCR signaling.
In Nature Chemical Biology on 1 February 2024 by Willette, B. K. A., Zhang, J. F., et al.
G-protein-coupled receptors (GPCRs) can initiate unique functional responses depending on the subcellular site of activation. Efforts to uncover the mechanistic basis of compartmentalized GPCR signaling have concentrated on the biochemical aspect of this regulation. Here we assess the biophysical positioning of receptor-containing endosomes as an alternative salient mechanism. We devise a strategy to rapidly and selectively redistribute receptor-containing endosomes 'on command' in intact cells without perturbing their biochemical composition. Next, we present two complementary optical readouts that enable robust measurements of bulk- and gene-specific GPCR/cyclic AMP (cAMP)-dependent transcriptional signaling with single-cell resolution. With these, we establish that disruption of native endosome positioning inhibits the initiation of the endosome-dependent transcriptional responses. Finally, we demonstrate a prominent mechanistic role of PDE-mediated cAMP hydrolysis and local protein kinase A activity in this process. Our study, therefore, illuminates a new mechanism regulating GPCR function by identifying endosome positioning as the principal mediator of spatially selective receptor signaling.
© 2023. The Author(s), under exclusive licence to Springer Nature America, Inc.
-
Cell Biology
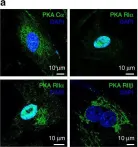
In Nat Commun on 5 September 2017 by Godbole, A., Lyga, S., et al.
Fig.4.A
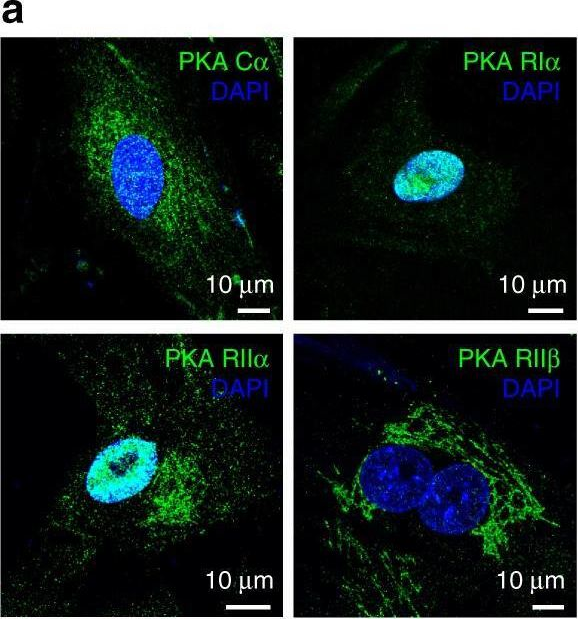
-
ICC-IF
-
Collected and cropped from Nature Communications by CiteAb, provided under a CC-BY license
Image 1 of 4
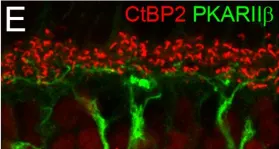
In PLoS One on 4 March 2017 by Puller, C., Arbogast, P., et al.
Fig.1.E
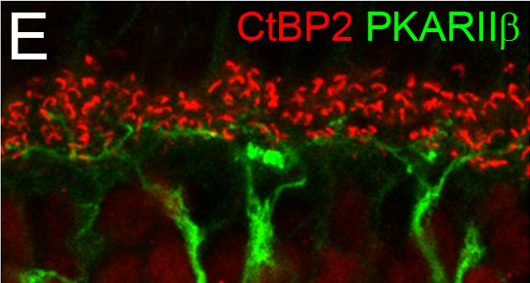
-
IHC-IF
-
Mus musculus (House mouse)
Collected and cropped from PLoS ONE by CiteAb, provided under a CC-BY license
Image 1 of 4
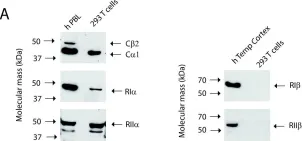
In BMC Biochem on 8 February 2011 by Stakkestad, Ø., Larsen, A. C., et al.
Fig.1.A
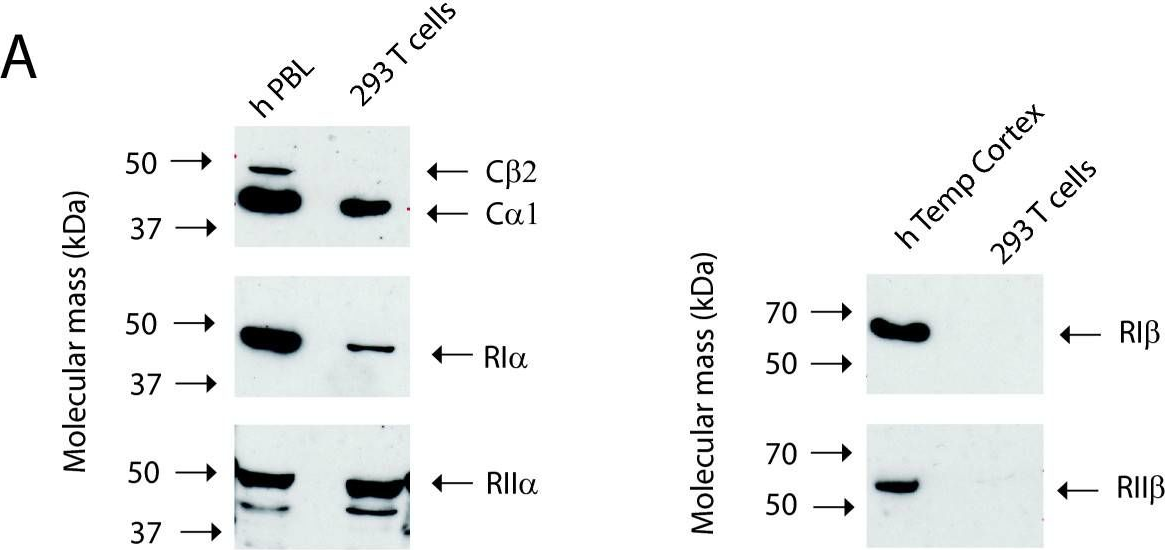
-
WB
-
Homo sapiens (Human)
Collected and cropped from BMC Biochemistry by CiteAb, provided under a CC-BY license
Image 1 of 4
In PLoS One on 30 April 2010 by Weisenhaus, M., Allen, M. L., et al.
Fig.2.C
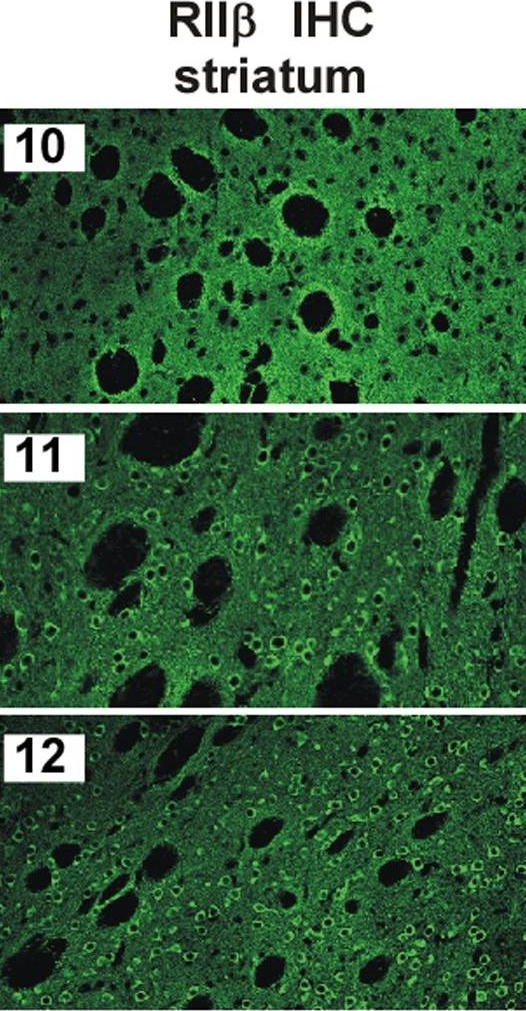
-
IHC
-
Mus musculus (House mouse)
Collected and cropped from PLoS ONE by CiteAb, provided under a CC-BY license
Image 1 of 4