The transmembrane protein β-amyloid precursor protein (APP) is central to the pathophysiology of Alzheimer's disease (AD). The β-amyloid hypothesis posits that aberrant processing of APP forms neurotoxic β-amyloid aggregates, which lead to the cognitive impairments observed in AD. Although numerous additional factors contribute to AD, there is a need to better understand the synaptic function of APP. We have found that Drosophila APP-like (APPL) has both shared and non-shared roles at the synapse with Kismet (Kis), a chromatin helicase binding domain (CHD) protein. Kis is the homolog of CHD7 and CHD8, both of which are implicated in neurodevelopmental disorders including CHARGE Syndrome and autism spectrum disorders, respectively. Loss of function mutations in kis and animals expressing human APP and BACE in their central nervous system show reductions in the glutamate receptor subunit, GluRIIC, the GTPase Rab11, and the bone morphogenetic protein (BMP), pMad, at the Drosophila larval neuromuscular junction (NMJ). Similarly, processes like endocytosis, larval locomotion, and neurotransmission are deficient in these animals. Our pharmacological and epistasis experiments indicate that there is a functional relationship between Kis and APPL, but Kis does not regulate appl expression at the larval NMJ. Instead, Kis likely influences the synaptic localization of APPL, possibly by promoting rab11 transcription. These data identify a potential mechanistic connection between chromatin remodeling proteins and aberrant synaptic function in AD.
Product Citations: 48
In International Journal of Molecular Sciences on 1 August 2024 by Hendricks, E. L., Linskey, N., et al.
-
ICC
In Journal of Extracellular Vesicles on 1 June 2024 by Mason, J. D., Marks, E., et al.
Exosomes are secreted vesicles made intracellularly in the endosomal system. We have previously shown that exosomes are not only made in late endosomes, but also in recycling endosomes marked by the monomeric G-protein Rab11a. These vesicles, termed Rab11a-exosomes, are preferentially secreted under nutrient stress from several cancer cell types, including HCT116 colorectal cancer (CRC) cells. HCT116 Rab11a-exosomes have particularly potent signalling activities, some mediated by the epidermal growth factor receptor (EGFR) ligand, amphiregulin (AREG). Mutant activating forms of KRAS, a downstream target of EGFR, are often found in advanced CRC. When absent, monoclonal antibodies, such as cetuximab, which target the EGFR and block the effects of EGFR ligands, such as AREG, can be administered. Patients, however, inevitably develop resistance to cetuximab, either by acquiring KRAS mutations or via non-genetic microenvironmental changes. Here we show that nutrient stress in several CRC cell lines causes the release of AREG-carrying Rab11a-exosomes. We demonstrate that while soluble AREG has no effect, much lower levels of AREG bound to Rab11a-exosomes from cetuximab-resistant KRAS-mutant HCT116 cells, can suppress the effects of cetuximab on KRAS-wild type Caco-2 CRC cells. Using neutralising anti-AREG antibodies and an intracellular EGFR kinase inhibitor, we show that this effect is mediated via AREG activation of EGFR, and not transfer of activated KRAS. Therefore, presentation of AREG on Rab11a-exosomes affects its ability to compete with cetuximab. We propose that this Rab11a-exosome-mediated mechanism contributes to the establishment of resistance in cetuximab-sensitive cells and may explain why in cetuximab-resistant tumours only some cells carry mutant KRAS.
© 2024 The Author(s). Journal of Extracellular Vesicles published by Wiley Periodicals LLC on behalf of International Society for Extracellular Vesicles.
-
Homo sapiens (Human)
-
Cancer Research
Preprint on BioRxiv : the Preprint Server for Biology on 30 March 2024 by Singh, P. J., Verma, B., et al.
Summary Secretory proteins frequently aggregate into non-soluble dense-core granules (DCGs) in recycling endosome-like compartments prior to release. By contrast, aberrantly processed Aβ-peptides derived from Amyloid Precursor Protein (APP) form pathological amyloidogenic aggregations in late-stage Alzheimer’s Disease (AD) after secretion. By examining living Drosophila prostate-like secondary cells, we show both APP and Aβ-peptides affect normal DCG biogenesis. These cells generate DCGs and secreted nanovesicles called Rab11-exosomes within enlarged recycling endosomes. The fly APP homologue, APP-like (APPL), associates with Rab11-exosomes and the compartmental limiting membrane, from where its extracellular domain controls protein aggregation. Proteolytic release of this membrane-associated domain permits aggregates to coalesce into a large central DCG. Mutant Aβ-peptide expression, like Appl loss-of-function, disrupts this assembly step and compartment motility, and increases lysosomal targeting, mirroring pathological events reported in early-stage AD. Our data therefore reveal a physiological role for APP in membrane-dependent protein aggregation, which when disrupted, rapidly triggers AD-relevant intracellular pathologies.
-
Cell Biology
FTIR Spectroscopy and Blood-Derived Extracellular Vesicles Duo in Alzheimer's Disease.
In Journal of Alzheimer's Disease : JAD on 15 March 2024 by Soares Martins, T., Ferreira, M., et al.
Alzheimer's disease (AD) diagnosis is difficult, and new accurate tools based on peripheral biofluids are urgently needed. Extracellular vesicles (EVs) emerged as a valuable source of biomarker profiles for AD, since their cargo is disease-specific and these can be easily isolated from easily accessible biofluids, as blood. Fourier Transform Infrared (FTIR) spectroscopy can be employed to analyze EVs and obtain the spectroscopic profiles from different regions of the spectra, simultaneously characterizing carbohydrates, nucleic acids, proteins, and lipids.
The aim of this study was to identify blood-derived EVs (bdEVs) spectroscopic signatures with AD discriminatory potential.
Herein, FTIR spectra of bdEVs from two biofluids (serum and plasma) and distinct sets of Controls and AD cases were acquired, and EVs' spectra analyzed.
Analysis of bdEVs second derivative peaks area revealed differences between Controls and AD cases in distinct spectra regions, assigned to carbohydrates and nucleic acids, amides, and lipids.
EVs' spectroscopic profiles presented AD discriminatory value, supporting the use of bdEVs combined with FTIR as a screening or complementary tool for AD diagnosis.
-
Cardiovascular biology
-
Neuroscience
Endolysosomal trafficking controls yolk granule biogenesis in vitellogenic Drosophila oocytes.
In PLoS Genetics on 1 February 2024 by Yu, Y., Chen, D., et al.
Endocytosis and endolysosomal trafficking are essential for almost all aspects of physiological functions of eukaryotic cells. As our understanding on these membrane trafficking events are mostly from studies in yeast and cultured mammalian cells, one challenge is to systematically evaluate the findings from these cell-based studies in multicellular organisms under physiological settings. One potentially valuable in vivo system to address this challenge is the vitellogenic oocyte in Drosophila, which undergoes extensive endocytosis by Yolkless (Yl), a low-density lipoprotein receptor (LDLR), to uptake extracellular lipoproteins into oocytes and package them into a specialized lysosome, the yolk granule, for storage and usage during later development. However, by now there is still a lack of sufficient understanding on the molecular and cellular processes that control yolk granule biogenesis. Here, by creating genome-tagging lines for Yl receptor and analyzing its distribution in vitellogenic oocytes, we observed a close association of different endosomal structures with distinct phosphoinositides and actin cytoskeleton dynamics. We further showed that Rab5 and Rab11, but surprisingly not Rab4 and Rab7, are essential for yolk granules biogenesis. Instead, we uncovered evidence for a potential role of Rab7 in actin regulation and observed a notable overlap of Rab4 and Rab7, two Rab GTPases that have long been proposed to have distinct spatial distribution and functional roles during endolysosomal trafficking. Through a small-scale RNA interference (RNAi) screen on a set of reported Rab5 effectors, we showed that yolk granule biogenesis largely follows the canonical endolysosomal trafficking and maturation processes. Further, the data suggest that the RAVE/V-ATPase complexes function upstream of or in parallel with Rab7, and are involved in earlier stages of endosomal trafficking events. Together, our study provides s novel insights into endolysosomal pathways and establishes vitellogenic oocyte in Drosophila as an excellent in vivo model for dissecting the highly complex membrane trafficking events in metazoan.
Copyright: © 2024 Yu et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
-
Genetics
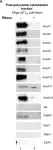
In Front Cell Dev Biol on 3 July 2023 by Patil, S. S., Panchal, V., et al.
Fig.1.A

-
WB
-
Collected and cropped from Front Cell Dev Biol by CiteAb, provided under a CC-BY license
Image 1 of 10
In Front Cell Dev Biol on 3 July 2023 by Patil, S. S., Panchal, V., et al.
Fig.1.B

-
WB
-
Collected and cropped from Front Cell Dev Biol by CiteAb, provided under a CC-BY license
Image 1 of 10
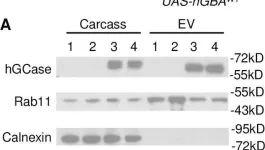
In PLoS Genet on 1 February 2021 by Jewett, K. A., Thomas, R. E., et al.
Fig.11.A

-
WB
-
Collected and cropped from PLoS Genet by CiteAb, provided under a CC-BY license
Image 1 of 10
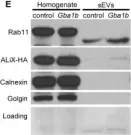
In PLoS Genet on 1 September 2018 by Thomas, R. E., Vincow, E. S., et al.
Fig.7.E

-
WB
-
Drosophila melanogaster (Fruit fly)
Collected and cropped from PLoS Genet by CiteAb, provided under a CC-BY license
Image 1 of 10
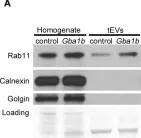
In PLoS Genet on 1 September 2018 by Thomas, R. E., Vincow, E. S., et al.
Fig.7.A

-
WB
-
Collected and cropped from PLoS Genet by CiteAb, provided under a CC-BY license
Image 1 of 10
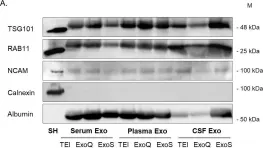
In PLoS One on 12 June 2018 by Soares Martins, T., Catita, J., et al.
Fig.7.A

-
WB
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 10
In Nat Commun on 24 July 2017 by Konitsiotis, A. D., Roßmannek, L., et al.
Fig.4.A

-
ICC-IF
-
Homo sapiens (Human)
Collected and cropped from Nat Commun by CiteAb, provided under a CC-BY license
Image 1 of 10
In PLoS One on 7 April 2017 by Oguchi, M. E., Noguchi, K., et al.
Fig.2.B

-
ICC-IF
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 10
In PLoS One on 7 April 2017 by Oguchi, M. E., Noguchi, K., et al.
Fig.2.C

-
WB
-
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 10
In PLoS One on 11 May 2011 by Zemskov, E. A., Mikhailenko, I., et al.
Fig.5.B

-
WB
-
Homo sapiens (Human)
Collected and cropped from PLoS One by CiteAb, provided under a CC-BY license
Image 1 of 10