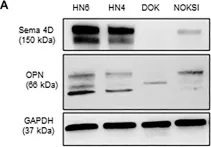
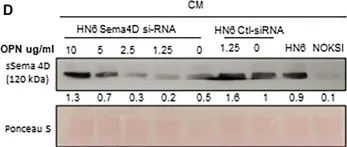
Head and neck squamous cell carcinoma (HNSCC) can be classified according to the histological inflammatory subtype (HIS) into inflamed (HIS-INF) or immune excluded (HIS-IE). HIS-IE was previously associated with higher levels of soluble Semaphorin 4D (HsS4D) in plasma, and higher transcriptional levels of osteopontin (OPN) in the tumor tissue, compared to HIS-INF. The goal of the current study is to investigate whether the HIS inflammatory subtype can be distinguished by a differential cytokine panel in peripheral blood. Retrospectively collected five HIS-INF and five HIS-IE tumor tissue with paired plasma were included in the study. Five healthy donors (HD) and five autoimmune/chronic inflammatory conditions (AI/CI) were controls. The ELISA-Luminex™ system was used to detect 40 traditional cytokines in plasma. Human cytokine array (104 cytokines) was used for the conditioned medium (CM) of the HNSCC HN6 cell line. Semaphorin 4D (Sema4D) siRNA and recombinant human osteopontin (rh-OPN) were used to investigate the effect of OPN on Sema4D expression. The HIS-IE cytokine profile was higher than HIS-INF but comparable to AI/CI. HIS-INF had the lowest cytokine levels. HIS-IE was differentially higher in IP-10 and IL8 compared to HD, while HIS-INF was higher in IL-10. Sema4D inhibition in HN6 resulted in a decrease of OPN in the CM of HN6, and treatment with rh-OPN rescued Sema4D in HN6 cell lysate and associated CM. In conclusion, the current work demonstrates a novel association between the HIS subtypes and a differential pattern of cytokine expression in plasma. These findings can open new avenues for HNSCC patient stratification and hence provide better personalized treatment.
© 2022 Ghita, Piperi, Atamas, Ord, Dyalram, Lubek and Younis.
Product Citations: 15
In Front Oral Health on 8 November 2022 by Ghita, I., Piperi, E., et al.
-
WB
-
Homo sapiens (Human)
-
Cancer Research
-
Immunology and Microbiology
In Frontiers in Immunology on 30 March 2021 by Younis, R. H., Ghita, I., et al.
Semaphorin 4D (Sema4D) is a glycoprotein that is expressed by several tumors and immune cells. It can function as a membrane bound protein or as a cleaved soluble protein (sSema4D). We sought to investigate the translational potential of plasma sSema4D as an immune marker in plasma of patients with head and neck squamous cell carcinoma (HNSCC). Paired peripheral blood and tumor tissue samples of 104 patients with HNSCC were collected at the same time point to allow for real time analysis. Scoring of the histological inflammatory subtype (HIS) was carried out using Sema4D immunohistochemistry on the tumor tissue. sSema4D was detected in plasma using direct ELISA assay. Defining elevated sSema4D as values above the 95th percentile in healthy controls, our data showed that sSema4D levels in plasma were elevated in 25.0% (95% CI, 16.7-34.9%) of the patients with HNSCC and showed significant association with HIS immune excluded (HIS-IE) (p = 0.007), Sema4D+ve tumor cells (TCs) (p = 0.018) and PD-L1+ve immune cells (ICs) (p = 0.038). A multi-variable logistic regression analysis showed that HIS was significantly (P = 0.004) associated with elevated sSema4D, an association not explained by available patient-level factors. Using the IO-360 nanoString platform, differential gene expression (DGE) analysis of 10 HNSCC tumor tissues showed that patients with high sSema4D in plasma (HsS4D) clustered as IFN-γ negative tumor immune signature and were mostly HIS-IE. The IC type in the HsS4D paired tumor tissue was predominantly myeloid, while the lymphoid compartment was higher in the low sSema4D (LsS4D). The Wnt signaling pathway was upregulated in the HsS4D group. Further analysis using the IO-360, 770 gene set, showed significant non-inflamed profile of the HsS4D tumors compared to the LsS4D. In conclusion, our data reveals an association between sSema4D and the histological inflammatory subtype.
Copyright © 2021 Younis, Ghita, Elnaggar, Chaisuparat, Theofilou, Dyalram, Ord, Davila, Tallon, Papadimitriou, Webb, Bentzen and Lubek.
-
IHC
-
Homo sapiens (Human)
-
Cancer Research
-
Immunology and Microbiology
Circulating Tumor Cells Exhibit Metastatic Tropism and Reveal Brain Metastasis Drivers.
In Cancer Discovery on 1 January 2020 by Klotz, R., Thomas, A., et al.
Hematogenous metastasis is initiated by a subset of circulating tumor cells (CTC) shed from primary or metastatic tumors into the blood circulation. Thus, CTCs provide a unique patient biopsy resource to decipher the cellular subpopulations that initiate metastasis and their molecular properties. However, one crucial question is whether CTCs derived and expanded ex vivo from patients recapitulate human metastatic disease in an animal model. Here, we show that CTC lines established from patients with breast cancer are capable of generating metastases in mice with a pattern recapitulating most major organs from corresponding patients. Genome-wide sequencing analyses of metastatic variants identified semaphorin 4D as a regulator of tumor cell transmigration through the blood-brain barrier and MYC as a crucial regulator for the adaptation of disseminated tumor cells to the activated brain microenvironment. These data provide the direct experimental evidence of the promising role of CTCs as a prognostic factor for site-specific metastasis. SIGNIFICANCE: Interests abound in gaining new knowledge of the physiopathology of brain metastasis. In a direct metastatic tropism analysis, we demonstrated that ex vivo-cultured CTCs from 4 patients with breast cancer showed organotropism, revealing molecular features that allow a subset of CTCs to enter and grow in the brain.This article is highlighted in the In This Issue feature, p. 1.
©2019 American Association for Cancer Research.
-
WB
-
Cancer Research
Regulatory sequence analysis of semaphorin 4D 5' non-coding region.
In Journal of Cancer on 12 March 2019 by Qiu, L., Jiang, H., et al.
Semaphorin 4D (Sema4D) has been proven to be one of the hypoxia effectors regulated by hypoxia inducible factor (HIF-1) in multiple cells, and play a role in angiogenesis like VEGF. However, the regulatory sequence characteristics of the Sema4D are not clarified. The possible hypoxia response element (HRE) sequences in 5' non-coding Region before ATG start codon of Sema4D were screened, followed by point mutagenesis and luciferase assay analysis. Sequencing and alignment of this region in 11 cancer cell lines and 4 normal cell lines were also performed, followed by cloning, mutation and luciferase assay analysis. The results showed that there were four possible HREs (HRE1-4) sequences in 1275bp range before ATG start codon. Among HRE1-4, HRE2 and HRE4 were functional HIF-1α binding sites. In addition, these two binding sites play different roles in the regulation of Sema4D expression in HUVEC and Caco-2 cells. There were three nucleotide variants (T471C/A600G/C862T) frequently detected in cancer cell lines. The site variation rates of T471C/A600G/C862T were 72.7%, 18.2%, and 72.7% in cancer cells respectively. Luciferase assays showed that T471C and C862T could significantly increase the expression efficiency of downstream target genes. Furthermore, secondary structure prediction showed that mutations at T471C and C862T apparently lead to change of the gene structure. Our study describes the sequence characteristics of 5' non-coding region of Sema4D, enhances our understanding of the regulatory mechanism of Sema4D and benefits the development of a possible anti-angiogenesis therapeutic strategy for malignancies.
-
WB
-
Homo sapiens (Human)
-
Cancer Research
In The FASEB Journal on 1 April 2018 by Zhou, Y. F., Li, Y. N., et al.
The inflammatory process in stroke is the major contributor to blood-brain barrier (BBB) breakdown. Previous studies indicated that semaphorin 4D (Sema4D), an axon guidance molecule, initiated inflammatory microglial activation and disrupted endothelial function in the CNS. However, whether Sema4D disrupts BBB integrity after stroke remains unclear. To study the effect of Sema4D on BBB disruption in stroke, rats were subjected to transient middle cerebral artery occlusion and targeted injection of lentivirus-mediated clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9 gene disruption of PlexinB1. We found that Sema4D synchronously increased with BBB permeability and accumulated in the perivascular area after stroke. Suppressing Sema4D/PlexinB1 signaling in the periinfarct cortex significantly decreased BBB permeability as detected by MRI and fibrin deposition, and thereby improved stroke outcome. In vitro, we confirmed that Sema4D disrupted BBB integrity and endothelial tight junctions. Moreover, we found that Sema4D induced pericytes to acquire a CD11b-positive phenotype and express proinflammatory cytokines. In addition, Sema4D inhibited AUF1-induced proinflammatory mRNA decay effect. Taken together, our data provides evidence that Sema4D disrupts BBB integrity and promotes an inflammatory response by binding to PlexinB1 in pericytes after transient middle cerebral artery occlusion. Our study indicates that Sema4D may be a novel therapeutic target for treatment in the acute phase of stroke.-Zhou, Y.-F., Li, Y.-N., Jin, H.-J., Wu, J.-H., He, Q.-W., Wang, X.-X., Lei, H., Hu, B. Sema4D/PlexinB1 inhibition ameliorates blood-brain barrier damage and improves outcome after stroke in rats.
-
IHC-IF
-
Rattus norvegicus (Rat)
-
Cardiovascular biology
In Front Oral Health on 8 November 2022 by Ghita, I., Piperi, E., et al.
Fig.5.A

-
WB
-
Collected and cropped from Front Oral Health by CiteAb, provided under a CC-BY license
Image 1 of 2
In Front Oral Health on 8 November 2022 by Ghita, I., Piperi, E., et al.
Fig.5.D

-
WB
-
Collected and cropped from Front Oral Health by CiteAb, provided under a CC-BY license
Image 1 of 2